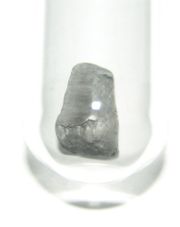
ثاليوم
 | |||||||||||||||||||||||||||||||||
| ثاليوم | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| المظهر | أبيض فضي | ||||||||||||||||||||||||||||||||
| الوزن الذري العياري Ar°(Tl) | |||||||||||||||||||||||||||||||||
| ثاليوم في الجدول الدوري | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| الرقم الذري (Z) | 81 | ||||||||||||||||||||||||||||||||
| المجموعة | 13 | ||||||||||||||||||||||||||||||||
| الدورة | period 6 | ||||||||||||||||||||||||||||||||
| المستوى الفرعي | p-block | ||||||||||||||||||||||||||||||||
| التوزيع الإلكتروني | [Xe] 4f14 5d10 6s2 6p1 | ||||||||||||||||||||||||||||||||
| الإلكترونات بالغلاف | 2, 8, 18, 32, 18, 3 | ||||||||||||||||||||||||||||||||
| الخصائص الطبيعية | |||||||||||||||||||||||||||||||||
| الطور at د.ح.ض.ق | solid | ||||||||||||||||||||||||||||||||
| نقطة الانصهار | 577 K (304 °س، 579 °F) | ||||||||||||||||||||||||||||||||
| نقطة الغليان | 1746 K (1473 °س، 2683 °ف) | ||||||||||||||||||||||||||||||||
| الكثافة حين يكون سائلاً (عند ن.إ.) | 11.22 ج/سم³ | ||||||||||||||||||||||||||||||||
| حرارة الانصهار | 4.14 kJ/mol | ||||||||||||||||||||||||||||||||
| حرارة التبخر | 165 kJ/mol | ||||||||||||||||||||||||||||||||
| السعة الحرارية المولية | 26.32 J/(mol·K) | ||||||||||||||||||||||||||||||||
ضغط البخار
| |||||||||||||||||||||||||||||||||
| الخصائص الذرية | |||||||||||||||||||||||||||||||||
| الكهرسلبية | مقياس پاولنگ: 1.62 | ||||||||||||||||||||||||||||||||
| طاقات التأين |
| ||||||||||||||||||||||||||||||||
| نصف القطر الذري | empirical: 170 pm | ||||||||||||||||||||||||||||||||
| نصف قطر التكافؤ | 145±7 pm | ||||||||||||||||||||||||||||||||
| نصف قطر ڤان در ڤالز | 196 pm | ||||||||||||||||||||||||||||||||
| خصائص أخرى | |||||||||||||||||||||||||||||||||
| البنية البلورية | hexagonal close-packed (hcp) | ||||||||||||||||||||||||||||||||
| سرعة الصوت قضيب رفيع | 818 م/ث (عند 20 °س) | ||||||||||||||||||||||||||||||||
| قضيب رفيع | 46.1 W/(m·K) | ||||||||||||||||||||||||||||||||
| التمدد الحراري | 29.9 µm/(m⋅K) (عند 25 °س) | ||||||||||||||||||||||||||||||||
| المقاومة الكهربائية | 0.18 µΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| الترتيب المغناطيسي | diamagnetic[1] | ||||||||||||||||||||||||||||||||
| القابلية المغناطيسية | −50.9×10−6 cm3/mol (298 K)[2] | ||||||||||||||||||||||||||||||||
| معامل يونگ | 8 GPa | ||||||||||||||||||||||||||||||||
| معامل القص | 2.8 GPa | ||||||||||||||||||||||||||||||||
| معاير الحجم | 43 GPa | ||||||||||||||||||||||||||||||||
| نسبة پواسون | 0.45 | ||||||||||||||||||||||||||||||||
| صلادة موز | 1.2 | ||||||||||||||||||||||||||||||||
| صلادة برينل | 26.5–44.7 MPa | ||||||||||||||||||||||||||||||||
| رقم كاس | 7440-28-0 | ||||||||||||||||||||||||||||||||
| التاريخ | |||||||||||||||||||||||||||||||||
| التسمية | after Greek thallos, green shoot or twig | ||||||||||||||||||||||||||||||||
| الاكتشاف | William Crookes (1861) | ||||||||||||||||||||||||||||||||
| أول عزل | Claude-Auguste Lamy (1862) | ||||||||||||||||||||||||||||||||
| نظائر الثاليوم | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
الثاليوم Thallium (IPA: /ˈθaliəm/) عنصر كيميائي في الجدول الدوري وله الرمز Tl ورقم ذري 81.[5] هذا الفلز الفقير القابل للتشكيل والرمادي الطري يشبه القصدير إلى أنه يتبقع عندما يتعرض للهواء. الثاليوم شديد السمية ويستعمل في سم الفئران والمبيدات الحشرية ولكن لاحتمال تسببه في السرطان (بالرغم من أن وكالة الحماية البيئية الأمريكية لا تصنفه كمسرطن)، هذا الاستخدام قد انخفض بشدة أو ألغي في العديد من الدول. ويستخدم أيضاً في مجسات الآشعة تحت الحمراء.[6] بل إنه قد استـُخدِم في بعض عمليات القتل، مما أكسبه الكـُنى "السم المفضل للمتخصص في التسميم" و "مسحوق الميراث" (بجانب الزرنيخ).
الثليوم عنصر كيميائي رمزه الكيميائي Tm. وهو واحد من عناصر الأتربة النادرة. وعدده الذري 69، ووزنه الذري 168,934. اكتشف العالم السويدي بيركليف الثليوم عام 1879م. يوجد الثليوم مع الأتربة النادرة الأخرى في معدن الجادولينيت، والأكسينيت، والزينوتيم، وغيرها. وأفضل الطرق لعزل الثليوم عن عناصر الأتربة الأخرى، هي عملية التبادل الأيوني، أو الاستخلاص بالمذيب. ينصهر الثاليوم في درجة حرارة 1,545°م، ويغلي في درجة حرارة 1,950°م، وكثافته 9,318جم/سم² عند درجة حرارة 25°م. يستخدم الثليوم المشع في وحدات الأشعة السينية المتنقلة. ومثل هذه الوحدات لا تحتاج إلى معدات كهربائية، بل تحتاج فقط إلى إعادة شحن بالثليوم، مرة واحدة فقط كل بضعة أشهر.
Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year.[7]
Thallium tends to form the +3 and +1 oxidation states. The +3 state resembles that of the other elements in group 13 (boron, aluminium, gallium, indium). However, the +1 state, which is far more prominent in thallium than the elements above it, recalls the chemistry of alkali metals and thallium(I) ions are found geologically mostly in potassium-based ores and (when ingested) are handled in many ways like potassium ions (K+) by ion pumps in living cells.
Commercially, thallium is produced not from potassium ores, but as a byproduct from refining of heavy-metal sulfide ores. Approximately 65% of thallium production is used in the electronics industry and the remainder is used in the pharmaceutical industry and in glass manufacturing.[8] It is also used in infrared detectors. The radioisotope thallium-201 (as the soluble chloride TlCl) is used in small amounts as an agent in a nuclear medicine scan, during one type of nuclear cardiac stress test.
Soluble thallium salts (many of which are nearly tasteless) are highly toxic and they were historically used in rat poisons and insecticides. Because of their nonselective toxicity, use of these compounds has been restricted or banned in many countries. Thallium poisoning usually results in hair loss. Because of its historic popularity as a murder weapon, thallium has gained notoriety as "the poisoner's poison" and "inheritance powder" (alongside arsenic).[9]
السمات
A thallium atom has 81 electrons, arranged in the electron configuration [Xe]4f145d106s26p1; of these, the three outermost electrons in the sixth shell are valence electrons. Due to the inert pair effect, the 6s electron pair is relativistically stabilised and it is more difficult to get these involved in chemical bonding than it is for the heavier elements. Thus, very few electrons are available for metallic bonding, similar to the neighboring elements mercury and lead. Thallium, then, like its congeners, is a soft, highly electrically conducting metal with a low melting point, of 304 °C.[10]
A number of standard electrode potentials, depending on the reaction under study,[11] are reported for thallium, reflecting the greatly decreased stability of the +3 oxidation state:[10]
| +0.73 | Tl3+ + 3 e− | ↔ Tl |
| −0.336 | Tl+ + e− | ↔ Tl |
Thallium is the first element in group 13 where the reduction of the +3 oxidation state to the +1 oxidation state is spontaneous under standard conditions.[10] Since bond energies decrease down the group, with thallium, the energy released in forming two additional bonds and attaining the +3 state is not always enough to outweigh the energy needed to involve the 6s-electrons.[12] Accordingly, thallium(I) oxide and hydroxide are more basic and thallium(III) oxide and hydroxide are more acidic, showing that thallium conforms to the general rule of elements being more electropositive in their lower oxidation states.[12]
Thallium is malleable and sectile enough to be cut with a knife at room temperature. It has a metallic luster that, when exposed to air, quickly tarnishes to a bluish-gray tinge, resembling lead. It may be preserved by immersion in oil. A heavy layer of oxide builds up on thallium if left in air. In the presence of water, thallium hydroxide is formed. Sulfuric and nitric acids dissolve thallium rapidly to make the sulfate and nitrate salts, while hydrochloric acid forms an insoluble thallium(I) chloride layer.[13]
النظائر
Thallium has 41 isotopes which have atomic masses that range from 176 to 216. 203Tl and 205Tl are the only stable isotopes and make up nearly all of natural thallium. The five short-lived isotopes 206Tl through 210Tl inclusive occur in nature, as they are part of the natural decay chains of heavier elements. 204Tl is the most stable radioisotope, with a half-life of 3.78 years.[14] It is made by the neutron activation of stable thallium in a nuclear reactor.[14][15] The most useful radioisotope, 201Tl (half-life 73 hours), decays by electron capture, emitting X-rays (~70–80 keV), and photons of 135 and 167 keV in 10% total abundance;[14] therefore, it has good imaging characteristics without an excessive patient-radiation dose. It is the most popular isotope used for thallium nuclear cardiac stress tests.[16]
المركبات
الثاليوم الثلاثي
Thallium(III) compounds resemble the corresponding aluminium(III) compounds. They are moderately strong oxidizing agents and are usually unstable, as illustrated by the positive reduction potential for the Tl3+/Tl couple. Some mixed-valence compounds are also known, such as Tl4O3 and TlCl2, which contain both thallium(I) and thallium(III). Thallium(III) oxide, Tl2O3, is a black solid which decomposes above 800 °C, forming the thallium(I) oxide and oxygen.[13]
The simplest possible thallium compound, thallane (TlH3), is too unstable to exist in bulk, both due to the instability of the +3 oxidation state as well as poor overlap of the valence 6s and 6p orbitals of thallium with the 1s orbital of hydrogen.[17] The trihalides are more stable, although they are chemically distinct from those of the lighter group 13 elements and are still the least stable in the whole group. For instance, thallium(III) fluoride, TlF3, has the β-BiF3 structure rather than that of the lighter group 13 trifluorides, and does not form the TlF−4 complex anion in aqueous solution. The trichloride and tribromide disproportionate just above room temperature to give the monohalides, and thallium triiodide contains the linear triiodide anion (I−3) and is actually a thallium(I) compound.[18] Thallium(III) sesquichalcogenides do not exist.[19]
الثاليوم الأحادي
The thallium(I) halides are stable. In keeping with the large size of the Tl+ cation, the chloride and bromide have the caesium chloride structure, while the fluoride and iodide have distorted sodium chloride structures. Like the analogous silver compounds, TlCl, TlBr, and TlI are photosensitive and display poor solubility in water.[20] The stability of thallium(I) compounds demonstrates its differences from the rest of the group: a stable oxide, hydroxide, and carbonate are known, as are many chalcogenides.[21]
The double salt Tl 4(OH) 2CO 3 has been shown to have hydroxyl-centred triangles of thallium, [Tl 3(OH)]2+ , as a recurring motif throughout its solid structure.[22]
The metalorganic compound thallium ethoxide (TlOEt, TlOC2H5) is a heavy liquid (ρ 3.49 g·cm−3, m.p. −3 °C),[23] often used as a basic and soluble thallium source in organic and organometallic chemistry.[24]
مركبات الثاليوم العضوي
Organothallium compounds tend to be thermally unstable, in concordance with the trend of decreasing thermal stability down group 13. The chemical reactivity of the Tl–C bond is also the lowest in the group, especially for ionic compounds of the type R2TlX. Thallium forms the stable [Tl(CH3)2]+ ion in aqueous solution; like the isoelectronic Hg(CH3)2 and [Pb(CH3)2]2+, it is linear. Trimethylthallium and triethylthallium are, like the corresponding gallium and indium compounds, flammable liquids with low melting points. Like indium, thallium cyclopentadienyl compounds contain thallium(I), in contrast to gallium(III).[25]
التاريخ
ثاليوم (باليونانية [θαλλός] Error: {{Lang}}: text has italic markup (help)، [thallos] Error: {{Lang}}: text has italic markup (help)، تعني "غصن أخضر")[26] اُكتـُشِف بمطيافية اللهب في 1861.[27] الاسم يأتي من خطوط الانبعاث الطيفية الخضراء للثاليوم.[28]
بعد نشر الطريقة المنقحة لمطيافية اللهب من قِبل روبرت بونزن وگوستاف كيرشوف[29] واكتشاف السيزيوم والروبيديوم في السنتين 1859 إلى 1860، أصبحت مطيافية اللهب طريقة مقبولة لتحديد مكونات المعادن والمواد الكيميائية. بدأ كل من وليام كروكس وكلود-أوگوست لامي في استخدام الطريقة الجديدة. وقد استخدمها وليام كروكس ليقوم بتحديدات طيفية للتلوريوم في مركبات السلنيوم المترسبة في lead chamber في مصنع لانتاج حمض الكبريتيك بالقرب من تيلكرود في جبال هارتس. وكان قد حصل على العينات لأبحاثه في سيانيد السلنيوم من أوگوست هوفمان قبل ذلك بأعوام.[30][31] وبحلول 1862، تمكن كروكس من عزل كميات صغيرة من العنصر الجديد وتحديد خصائص بعض المركبات.[32] استخدم كلود-أوگوست لامي مطياف مشابه لمطياف كروكس لتحديد تركيب مادة محتوية على السلنيوم، ترسبت أثناء انتاج حمض الكبريتيك من الپيريت. كما لاحظ الخط الأخضر الجديد في الأطياف واستنتج أن عنصراً جديداً كان موجوداً. وقد تسلم لامي هذه المادة من مصنع حمض الكبريتيك الذي يملكه صديقه فريد كولمان وكان هذا الناتج الجانبي متوافراً بكميات كبيرة. بدأ لامي في عزل العنصر الجديد من هذا المصدر.[33] وحقيقة أن لامي كان قادراً على العمل على كميات وفيرة من الثاليوم مكـَّنه من تحديد خصائص مركبات عديدة وبالاضافة لذلك فقد أعد سبيكة صغيرة من الثاليوم الفلزي حضـَّرها بالتحليل الكهربائي لأملاح الثاليوم.
As both scientists discovered thallium independently and a large part of the work, especially the isolation of the metallic thallium was done by Lamy, Crookes tried to secure his own priority on the work. Lamy was awarded a medal at the International Exhibition in London 1862: For the discovery of a new and abundant source of thallium and after heavy protest Crookes also received a medal: thallium, for the discovery of the new element. The controversy between both scientists continued through 1862 and 1863. Most of the discussion ended after Crookes was elected Fellow of the Royal Society in June 1863.[34][35]
The dominant use of thallium was the use as poison for rodents. After several accidents the use as poison was banned in the United States by Presidential Executive Order 11643 in February 1972. In subsequent years several other countries also banned its use.[36]
التواجد والإنتاج
Thallium concentration in the Earth's crust is estimated to be 0.7 mg/kg,[37] mostly in association with potassium-based minerals in clays, soils, and granites. The major source of thallium for practical purposes is the trace amount that is found in copper, lead, zinc, and other heavy-metal-sulfide ores.[38][39]
Thallium is found in the minerals crookesite TlCu7Se4, hutchinsonite TlPbAs5S9, and lorándite TlAsS2.[40] Thallium also occurs as a trace element in iron pyrite, and thallium is extracted as a by-product of roasting this mineral for the production of sulfuric acid.[8][41]
Thallium can also be obtained from the smelting of lead and zinc ores. Manganese nodules found on the ocean floor contain some thallium.[42] In addition, several other thallium minerals, containing 16% to 60% thallium, occur in nature as complexes of sulfides or selenides that primarily contain antimony, arsenic, copper, lead, and silver. These minerals are rare, and have had no commercial importance as sources of thallium.[37] The Allchar deposit in southern North Macedonia was the only area where thallium was actively mined. This deposit still contains an estimated 500 tonnes of thallium, and it is a source for several rare thallium minerals, for example lorándite.[43]
The United States Geological Survey (USGS) estimates that the annual worldwide production of thallium is 10 metric tonnes as a by-product from the smelting of copper, zinc, and lead ores.[37] Thallium is either extracted from the dusts from the smelter flues or from residues such as slag that are collected at the end of the smelting process.[37] The raw materials used for thallium production contain large amounts of other materials and therefore a purification is the first step. The thallium is leached either by the use of an alkali or sulfuric acid from the material. The thallium is precipitated several times from the solution to remove impurities. At the end it is converted to thallium sulfate and the thallium is extracted by electrolysis on platinum or stainless steel plates.[41] The production of thallium decreased by about 33% in the period from 1995 to 2009 – from about 15 metric tonnes to about 10 tonnes. Since there are several small deposits or ores with relatively high thallium content, it would be possible to increase the production if a new application, such as a thallium-containing high-temperature superconductor, becomes practical for widespread use outside of the laboratory.[44]
التطبيقات
الاستخدامات التاريخية
The odorless and tasteless thallium sulfate was once widely used as rat poison and ant killer. Since 1972 this use has been prohibited in the الولايات المتحدة due to safety concerns.[36] Many other countries followed this example in the following years.[8] Thallium salts were used in the treatment of ringworm, other skin infections and to reduce the night sweating of tuberculosis patents. However this use has been limited due to their narrow therapeutic index, and the development of more-advanced medicines for these conditions.[45][46][47]
البصريات
Thallium(I) bromide and thallium(I) iodide crystals have been used as infrared optical materials, because they are harder than other common infrared optics, and because they have transmission at significantly longer wavelengths. The trade name KRS-5 refers to this material.[48] Thallium oxide has been used to manufacture glasses that have a high index of refraction. Combined with sulfur or selenium and arsenic, thallium has been used in the production of high-density glasses that have low melting points in the range of 125 and 150 °C. These glasses have room temperature properties that are similar to ordinary glasses and are durable, insoluble in water and have unique refractive indices.[49]
الإلكترونيات
الموصلية الكهربائية لكبريتيدات الثاليوم الأحادي تتغير بالتعرض لضوء آشعة تحت الحمراء وهو ما يجعل هذا المركب مفيداً في photoresistors.[45] وقد اُستخدم سلنيد الثاليوم في بولومتر للكشف عن الآشعة تحت الحمراء.[50] Doping أشباه موصلات السلنيوم يالثاليوم يحسن أداءهم، ولذلك يستخدَم بكميات ضئيلة جداً في مقومات السلنيوم.[45] واستخدام آخر للغمس في الثاليوم هو بلورات يوديد الصوديوم في أجهزة الكشف عن اشعاع گاما. وفي هؤلاء، فإن بلورات يوديد الصوديوم are doped بكمية صغيرة من الثاليوم لتحسين كفاءتهم كمولدات scintillation.[51] بعض الأقطاب الكهربائية في محللات الأكسجين المذاب تحتوي على الثاليوم.[8]
الموصلية الفائقة عند درجات الحرارة العالية
الاستخدامات الطبية
Before the widespread application of technetium-99m in nuclear medicine, the radioactive isotope thallium-201, with a half-life of 73 hours, was the main substance for nuclear diagnostics. The nuclide is still used for stress tests for risk stratification in patients with coronary artery disease (CAD).[52] This isotope of thallium can be generated using a transportable generator which is similar to the technetium cow.[53] The generator contains lead-201 (half life 9.33 hours) which decays by electron capture to the thallium-201. The lead-201 can be produced in a cyclotron by the bombardment of thallium with protons or deuterons by the (p,3n) and (d,4n) reactions.[54][55]
اختبار المجهود بالثاليوم
A thallium stress test is a form of scintigraphy, where amount of thallium in tissues correlates with tissue blood supply. Viable cardiac cells have normal Na+/K+ ion exchange pumps. Thallium binds the K+ pumps and is transported into the cells. Exercise or dipyridamole induces widening (vasodilation) of normal coronary arteries. This produces coronary steal from areas where arteries are maximally dilated. Areas of infarct or ischemic tissue will remain "cold". Pre- and post-stress thallium may indicated areas which will benefit from myocardial revascularization. Redistribution indicates the existence of coronary steal and the presence of ischemic coronary artery disease.[56]
استخدامات أخرى
السمية
| المخاطر | |
|---|---|
| ن.م.ع. مخطط تصويري |   
|
| ن.م.ع. كلمة الاشارة | Danger |
| H300, H330, H373, H413 | |
| P260, P264, P284, P301, P310[57] | |
| NFPA 704 (معيـَّن النار) | |
الثاليوم ومركباته شديدو السمية، ويجب تداولهم بحذر كبير. واتصاله بالبشرة خطير، ويجب توفير تهوية مناسبة أثناء صهر هذا الفلز. مركبات الثاليوم (الأحادي) لها قابلية عالية للذوبان في الماء ويتم امتصاصها بسرعة عبر مسام الجلد. والتعرض لهم يجب ألا يتعدى 0.1 مج لكل م2 من البشرة في متوسط موزَّع لفترة طولها 8-ساعات (لأسبوع عمل طوله 40-ساعة عمل). ويـُعتقد أن الثاليوم مسرطن للبشر.[58] لزمن طويل كانت مركبات الثاليوم متوافرة كسم فئران. هذه الحقيقة بالاضافة لكونه سهل الذوبان في الماء وكونه عديم الطعم أدى إلى حالات تسمم متعددة غير مقصودة أو مقصودة إجرامياً.[35]
Contact with skin is dangerous, and adequate ventilation is necessary when melting this metal. Thallium(I) compounds have a high aqueous solubility and are readily absorbed through the skin, and care should be taken to avoid this route of exposure, as cutaneous absorption can exceed the absorbed dose received by inhalation at the permissible exposure limit (PEL).[59] Exposure by inhalation cannot safely exceed 0.1 mg/m2 in an eight-hour time-weighted average (40-hour work week).[60] The Centers for Disease Control and Prevention (CDC) states, "Thallium is not classifiable as a carcinogen, and it is not suspected to be a carcinogen. It is unknown whether chronic or repeated exposure to thallium increases the risk of reproductive toxicity or developmental toxicity. Chronic high level exposure to thallium through inhalation has been reported to cause nervous system effects, such as numbness of fingers and toes."[61] For a long time thallium compounds were readily available as rat poison. This fact and that it is water-soluble and nearly tasteless led to frequent intoxication caused by accident or criminal intent.[35]
One of the main methods of removing thallium (both radioactive and stable) from humans is to use Prussian blue, a material which absorbs thallium.[62] Up to 20 grams per day of Prussian blue is fed by mouth to the patient, and it passes through their digestive system and comes out in their stool. Hemodialysis and hemoperfusion are also used to remove thallium from the blood serum. At later stages of the treatment, additional potassium is used to mobilize thallium from the tissues.[63][64]
According to the United States Environmental Protection Agency (EPA), artificially-made sources of thallium pollution include gaseous emission of cement factories, coal-burning power plants, and metal sewers. The main source of elevated thallium concentrations in water is the leaching of thallium from ore processing operations.[39][65]
تسميم ياسر عرفات
وفي 10 يناير 2011، اتهم بسام أبو شريف، مساعد أبي عمار، إسرائيل بإغتيال ياسر عرفات باستخدام الثاليوم. وقال أن لديه تقريراً من كبير خبراء السموم الجنائية بإنجلترة، دون ذكر اسمه ولا اسم الجهة التي قامت بالبحث أو التي طلبته.[66]
العلاج وازالة التلوث الداخلي
أحد الطرق الرئيسة لإزالة الثاليوم (المشع والعادي) من الجسم البشري هي باستخدام الأزرق الپروسي، الذي هو مادة تمتص الثاليوم.[67] فحتى 20 گرام في اليوم من الأزرق البروسي يـُعطى بالفم للشخص، فتمر عبر الجهاز الهضمي وتخرج في البراز. الديال الدموي Hemodialysis والنضح الدموي hemoperfusion يـُستخدما أيضاً لازالة الثاليوم من مصل الدم. وفي مرحلة متأخرة من المعالجة، يُستخدام پوتاسيوم اضافي لتحريك الثاليوم من الأنسجة.[68][69]
تلوث الثاليوم
According to the United States Environmental Protection Agency (EPA), artificially-made sources of thallium pollution include gaseous emission of cement factories, coal-burning power plants, and metal sewers. The main source of elevated thallium concentrations in water is the leaching of thallium from ore processing operations.[39][70]
انظر أيضاً
- مركبات الثاليوم
- The entry for thallium at fictional applications of real materials
- اختبار المجهود بالثاليوم Thallium Stress Test
المصادر
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ أ ب Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ thallium, Los Alamos National Laboratory. Retrieved November 21, 2006.
- ^
Nayer, P. S. "Thallium selenide infrared detector". Smithsonian/NASA ADS Physics Abstract Service. Retrieved 2006-11-25.
{{cite web}}: Cite has empty unknown parameters:|accessyear=,|month=, and|accessmonthday=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ The Mining and Smelting Magazine Archived 2021-02-24 at the Wayback Machine. Ed. Henry Curwen Salmon. Vol. iv, July–Dec 1963, p. 87.
- ^ أ ب ت ث "Chemical fact sheet – Thallium". Spectrum Laboratories. April 2001. Archived from the original on 2008-02-21. Retrieved 2008-02-02.
- ^ Hasan, Heather (2009). The Boron Elements: Boron, Aluminum, Gallium, Indium, Thallium. Rosen Publishing Group. p. 14. ISBN 978-1-4358-5333-1.
- ^ أ ب ت Greenwood and Earnshaw, pp. 222–224
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 8.20. ISBN 1439855110.
- ^ أ ب Greenwood and Earnshaw, pp. 224–7
- ^ أ ب Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Thallium". Lehrbuch der Anorganischen Chemie (in الألمانية) (91–100 ed.). Walter de Gruyter. pp. 892–893. ISBN 978-3-11-007511-3.
- ^ أ ب ت Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A 729: 3–128, doi:, Bibcode: 2003NuPhA.729....3A, https://hal.archives-ouvertes.fr/in2p3-00020241/document
- ^ "Manual for reactor produced radioisotopes" (PDF). International Atomic Energy Agency. 2003. Archived (PDF) from the original on 2011-05-21. Retrieved 2010-05-13.
- ^ Maddahi, Jamshid; Berman, Daniel (2001). "Detection, Evaluation, and Risk Stratification of Coronary Artery Disease by Thallium-201 Myocardial Perfusion Scintigraphy 155". Cardiac SPECT imaging (2nd ed.). Lippincott Williams & Wilkins. pp. 155–178. ISBN 978-0-7817-2007-6. Archived from the original on 2017-02-22. Retrieved 2016-09-26.
- ^ Andrew, L.; Wang, X. (2004). "Infrared Spectra of Thallium Hydrides in Solid Neon, Hydrogen, and Argon". J. Phys. Chem. A. 108 (16): 3396–3402. Bibcode:2004JPCA..108.3396W. doi:10.1021/jp0498973.
- ^ Greenwood and Earnshaw, p. 239
- ^ Greenwood and Earnshaw, p. 254
- ^ Greenwood and Earnshaw, p. 241
- ^ Greenwood and Earnshaw, pp. 246–7
- ^ Siidra, Oleg I.; Britvin, Sergey N.; Krivovichev, Sergey V. (2009). "Hydroxocentered [(OH)Tl 3]2+ triangle as a building unit in thallium compounds: synthesis and crystal structure of Tl 4(OH) 2CO 3". Z. Kristallogr. 224 (12): 563–567. Bibcode:2009ZK....224..563S. doi:10.1524/zkri.2009.1213. S2CID 97334707.
- ^ Handbook of inorganic compounds. Perry, Dale L., Phillips, Sidney L. Boca Raton: CRC Press. 1995. ISBN 0-8493-8671-3. OCLC 32347397.
{{cite book}}: CS1 maint: others (link) - ^ Frank, Scott A.; Chen, Hou; Kunz, Roxanne K.; Schnaderbeck, Matthew J.; Roush, William R. (2000-08-01). "Use of Thallium(I) Ethoxide in Suzuki Cross Coupling Reactions". Organic Letters. 2 (17): 2691–2694. doi:10.1021/ol0062446. ISSN 1523-7060. PMID 10990429.
- ^ Greenwood and Earnshaw, pp. 262–4
- ^ Liddell & Scott, A Greek-English Lexicon, sub θαλλος
- ^ Thallium was discovered both by William Crookes and by Claude Auguste Lamy, working independently. See: (1) William Crookes (March 30, 1861) "On the existence of a new element, probably of the sulphur group," Chemical News, vol. 3, pages 193-194; reprinted in: Philosophical Magazine, vol. 21, pages 301-305 (April 1861); (2) William Crookes (May 18, 1861) "Further remarks on the supposed new metalloid," Chemical News, vol. 3, page 303; (3) William Crookes (June 19, 1862) "Preliminary researches on thallium," Proceedings of the Royal Society of London, vol. 12, pages 150-159. See also: A. Lamy (May 16, 1862) "De l'existencè d'un nouveau métal, le thallium," Comptes Rendus, vol. 54, pages 1255-1262.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. XIII. Supplementary note on the discovery of thallium". Journal of Chemical Education. 9: 2078. doi:10.1021/ed009p2078.
- ^ G. Kirchhoff, R. Bunsen (1861). "Chemische Analyse durch Spectralbeobachtungen". Annalen der Physik und Chemie. 189 (7): 337–381. doi:10.1002/andp.18611890702.
- ^ Crookes, William (1862 - 1863). "Preliminary Researches on Thallium". Proceedings of the Royal Society of London,. 12: 150–159. doi:10.1098/rspl.1862.0030.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: extra punctuation (link) - ^ Crookes, William (1863). "On Thallium". Philosophical Transactions of the Royal Society of London,. 153: 173–192. doi:10.1098/rstl.1863.0009.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ DeKosky, Robert K. (1973). "Spectroscopy and the Elements in the Late Nineteenth Century: The Work of Sir William Crookes". The British Journal for the History of Science. 6 (4): 400–423. doi:10.1017/S0007087400012553.
- ^ Lamy, Claude-Auguste (1862). "De l'existencè d'un nouveau métal, le thallium". Comptes Rendus: 1255–.
- ^ James, Frank A. J. L. (1984). "Of 'Medals and Muddles' the Context of the Discovery of Thallium: William Crookes's Early". Notes and Records of the Royal Society of London. 39 (1): 65–90. doi:10.1098/rsnr.1984.0005. JSTOR 531576.
- ^ أ ب ت Emsley, John (2006). "Thallium". The Elements of Murder: A History of Poison. Oxford University Press. pp. 326–327. ISBN 978-0-19-280600-0. Archived from the original on 2020-03-07. Retrieved 2016-09-26.
- ^ أ ب Staff of the Nonferrous Metals Division (1972). "Thallium". Minerals yearbook metals, minerals, and fuels. Vol. 1. United States Geological Survey. p. 1358. Archived from the original on 2014-03-22. Retrieved 2010-06-01.
- ^ أ ب ت ث Guberman, David E. "Mineral Commodity Summaries 2010: Thallium" (PDF). United States Geological Survey. Archived (PDF) from the original on 2010-07-15. Retrieved 2010-05-13.
- ^ Zitko, V.; Carson, W. V.; Carson, W. G. (1975). "Thallium: Occurrence in the environment and toxicity to fish". Bulletin of Environmental Contamination and Toxicology. 13 (1): 23–30. Bibcode:1975BuECT..13...23Z. doi:10.1007/BF01684859. PMID 1131433. S2CID 40955658.
- ^ أ ب ت Peter, A.; Viraraghavan, T. (2005). "Thallium: a review of public health and environmental concerns". Environment International. 31 (4): 493–501. Bibcode:2005EnInt..31..493P. doi:10.1016/j.envint.2004.09.003. PMID 15788190.
- ^ Shaw, D. (1952). "The geochemistry of thallium". Geochimica et Cosmochimica Acta. 2 (2): 118–154. Bibcode:1952GeCoA...2..118S. doi:10.1016/0016-7037(52)90003-3.
- ^ أ ب Downs, Anthony John (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. pp. 90 and 106. ISBN 978-0-7514-0103-5. Archived from the original on 2017-02-22. Retrieved 2016-09-26.
- ^ Rehkamper, M.; Nielsen, Sune G. (2004). "The mass balance of dissolved thallium in the oceans". Marine Chemistry. 85 (3–4): 125–139. Bibcode:2004MarCh..85..125R. doi:10.1016/j.marchem.2003.09.006.
- ^ Jankovic, S. (1988). "The Allchar Tl–As–Sb deposit, Yugoslavia and its specific metallogenic features". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 271 (2): 286. Bibcode:1988NIMPA.271..286J. doi:10.1016/0168-9002(88)90170-2.
- ^ Smith, Gerald R. "Mineral commodity summaries 1996: Thallium" (PDF). United States Geological Survey. Archived (PDF) from the original on 2010-05-29. Retrieved 2010-05-13.
- ^ أ ب ت Hammond, C. R. The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304857.
- ^ Percival, G. H. (1930). "The Treatment of Ringworm of The Scalp with Thallium Acetate". British Journal of Dermatology. 42: 59–69. doi:10.1111/j.1365-2133.1930.tb09395.x.
- ^ Galvanarzate, S; Santamaría, A (1998). "Thallium toxicity". Toxicology Letters. 99 (1): 1–13. doi:10.1016/S0378-4274(98)00126-X. PMID 9801025.
- ^ Rodney, William S.; Malitson, Irving H. (1956). "Refraction and Dispersion of Thallium Bromide Iodide". Journal of the Optical Society of America. 46: 338–346. doi:10.1364/JOSA.46.000956.
- ^ Kokorina, Valentina F. (1996). Glasses for infrared optics. CRC Press. ISBN 9780849337857.
- ^ Nayer, P. S, Hamilton, O. (1977). "Thallium selenide infrared detector". Appl. Opt. 16: 2942. doi:10.1364/AO.16.002942.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hofstadter, Robert (1949). "The Detection of Gamma-Rays with Thallium-Activated Sodium Iodide Crystals". Physical Review. 75: 796–810. doi:10.1103/PhysRev.75.796.
- ^ Jain, Diwakar; Zaret, Barry L. (2005). "Nuclear Imaging in Cardiovascular Medicine". In Clive Rosendorff (ed.). Essential cardiology: principles and practice (2 ed.). Humana Press. pp. 221–222. ISBN 9781588293701.
- ^ Lagunas-Solar, M. C. (1982). Abstract "An integrally shielded transportable generator system for thallium-201 production". International Journal of Applied Radiation Isotopes. 33 (12): 1439–1443. doi:10.1016/0020-708X(82)90183-1.
{{cite journal}}: Check|url=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Thallium-201 production from Harvard Medical School's Joint Program in Nuclear Medicine
- ^ Lebowitz, E.; Greene, M. W.; Fairchild, R.; Bradley-Moore, P. R.; Atkins, H. L.; Ansari, A. N.; Richards, P.; Belgrave, E. (1975). "Thallium-201 for Medical Use". The Journal of Nuclear Medicine. 16 (2): 151–5. PMID 1110421.
- ^ George J. Taylor (2004). Primary Care Cardiology. Wiley-Blackwell. p. 100. ISBN 1405103868.
- ^ "Thallium 277932". Sigma-Aldrich. Archived from the original on 2018-10-02. Retrieved 2018-10-02.
- ^ "Biology of Thallium". webelemnts. Retrieved 2008-11-11.
- ^ "Surface Contamination - Overview | Occupational Safety and Health Administration". www.osha.gov. Retrieved 2023-02-12.
- ^ Chemical Sampling Information | Thallium, soluble compounds (as Tl) Archived 2014-03-22 at the Wayback Machine. Osha.gov. Retrieved on 2013-09-05.
- ^ "CDC – The Emergency Response Safety and Health Database: Systemic Agent: THALLIUM – NIOSH". www.cdc.gov. Archived from the original on 2019-11-15. Retrieved 2019-12-11.
- ^ Yang, Yongsheng; Faustino, Patrick J.; Progar, Joseph J.; et al. (2008). "Quantitative determination of thallium binding to ferric hexacyanoferrate: Prussian blue". International Journal of Pharmaceutics. 353 (1–2): 187–194. doi:10.1016/j.ijpharm.2007.11.031. PMID 18226478. Archived from the original on 2020-03-15. Retrieved 2019-07-01.
- ^ Prussian blue fact sheet Archived 2013-10-20 at the Wayback Machine. US Centers for Disease Control and Prevention.
- ^ Malbrain, Manu L. N. G.; Lambrecht, Guy L. Y.; Zandijk, Erik; Demedts, Paul A.; Neels, Hugo M.; Lambert, Willy; De Leenheer, André P.; Lins, Robert L.; Daelemans, Ronny (1997). "Treatment of Severe Thallium Intoxication". Clinical Toxicology. 35 (1): 97–100. doi:10.3109/15563659709001173. PMID 9022660.
- ^ "Factsheet on: Thallium" (PDF). US Environmental Protection Agency. Archived (PDF) from the original on 2012-01-11. Retrieved 2009-09-15.
- ^ "اكتشاف السم الذي قتل عرفات". [[]]. 2011-01-10.
- ^ Yang, Y; Faustino, P; Progar, J; Brownell, C; Sadrieh, N; May, J; Leutzinger, E; Place, D; Duffy, E (2008). "Quantitative determination of thallium binding to ferric hexacyanoferrate: Prussian blue☆". International Journal of Pharmaceutics. 353 (1–2): 187–94. doi:10.1016/j.ijpharm.2007.11.031. PMID 18226478.
- ^ Prussian blue fact sheet from the Centers for Disease Control and Prevention
- ^ Malbrain, Manu L. N. G. (1997). "Treatment of Severe Thallium Intoxication". Clinical Toxicology. 35 (1): 97–100. doi:10.3109/15563659709001173. PMID 9022660.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ "Factsheet on: Thallium" (PDF). US Environmental Protection Agency. Archived (PDF) from the original on 2012-01-11. Retrieved 2009-09-15.
وصلات خارجية
- WebElements.com — Thallium
- pure Thallium >=99,99% picture in the element collection from Heinrich Pniok
- Toxicity, Thallium
- NLM Hazardous Substances Databank – Thallium, Elemental
- ATSDR - ToxFAQs
| الجدول الدوري | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||
- CS1 errors: unsupported parameter
- CS1 الألمانية-language sources (de)
- CS1 maint: others
- CS1 errors: URL
- Short description is different from Wikidata
- Pages using infobox element with unknown parameters
- Articles containing Greek-language text
- Articles with hatnote templates targeting a nonexistent page
- Lang and lang-xx template errors
- Chembox container only
- عناصر كيميائية
- ثاليوم
- فلزات ضعيفة
- علم السموم