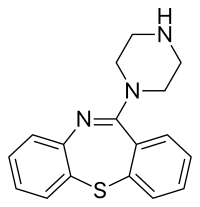
كويتياپين
 | |
 | |
| البيانات السريرية | |
|---|---|
| النُطق | /kwᵻˈtaɪ.əpiːn/ kwi-TY-ə-peen |
| الأسماء التجارية | Seroquel, Temprolide, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698019 |
| License data |
|
| فئة السلامة أثناء الحمل |
|
| مسارات الدواء | By mouth |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | 100%[1] |
| ارتباط الپروتين | 83%[2] |
| الأيض | Liver via CYP3A4-catalysed sulfoxidation to its active metabolite norquetiapine (N-desalkylquetiapine)[5] |
| Elimination half-life | 7 hours (parent compound); 9–12 hours (active metabolite, norquetiapine)[2][3] |
| الإخراج | Kidney (73%), faeces (20%)[1][2][3][4] |
| المعرفات | |
| رقم CAS | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.193 |
| Chemical and physical data | |
| التركيب | C21H25N3O2S |
| الكتلة المولية | 383.5099 g/mol |
| 3D model (JSmol) | |
| Solubility in water | 3.29 mg/mL (20 °C) |
| (verify) | |
كويتاپين، يُباع تحت الاسم التجاري سروكويل Seroquel، is an atypical antipsychotic used for the treatment of schizophrenia, bipolar disorder, and major depressive disorder.[6][7] Despite being widely used as a sleep aid due its sedating effect, the benefits of such use do not appear to generally outweigh the side effects.[8] It is taken by mouth.[6]
Common side effects include sleepiness, constipation, weight gain, and dry mouth.[6] Other side effects include low blood pressure with standing, seizures, a prolonged erection, high blood sugar, tardive dyskinesia, and neuroleptic malignant syndrome.[6] In older people with dementia, its use increases the risk of death.[6] Use in the third trimester of pregnancy may result in a movement disorder in the baby for some time after birth.[6] Quetiapine is believed to work by blocking a number of receptors including serotonin and dopamine.[6]
Quetiapine was developed in 1985 and approved for medical use in the United States in 1997.[6][9] It is available as a generic medication.[10] In the United States, the wholesale cost is about US$12 per month as of 2017.[11] In the United Kingdom, a month's supply costs the NHS about £60 as of 2017.[10] In 2016, it was the 86th most prescribed medication in the United States, with more than 8 million prescriptions.[12]
الاستخدامات الطبية
Quetiapine is primarily used to treat schizophrenia or bipolar disorder.[13] Quetiapine targets both positive and negative symptoms of schizophrenia.[14]
الفصام
A 2013 Cochrane review compared quetiapine to typical antipsychotics:
| Summary | ||||
|---|---|---|---|---|
| Quetiapine may not differ from typical antipsychotics in the treatment of positive symptoms, general psychopathology, and negative symptoms. However, it causes fewer adverse effects in terms of abnormal ECG, extrapyramidal effects, abnormal prolactin levels and weight gain.[15] | ||||
|
In a 2013 comparison of 15 antipsychotics in effectiveness in treating schizophrenia, quetiapine demonstrated standard effectiveness. It was 13-16% more effective than ziprasidone, chlorpromazine, and asenapine and approximately as effective as haloperidol and aripiprazole.[16]
There is tentative evidence of the benefit of quetiapine versus placebo in schizophrenia; however, definitive conclusions are not possible due to the high rate of attrition in trials (greater than 50%) and the lack of data on economic outcomes, social functioning, or quality of life.[17]
It is debatable whether, as a class, typical or atypical antipsychotics are more effective.[18] Both have equal drop-out and symptom relapse rates when typicals are used at low to moderate dosages.[19] While quetiapine has lower rates of extrapyramidal side effects, there is greater sleepiness and rates of dry mouth.[17]
A Cochrane review comparing quetiapine to other atypical antipsychotic agents tentatively concluded that it may be less efficacious than olanzapine and risperidone; produce fewer movement related side effects than paliperidone, aripiprazole, ziprasidone, risperidone and olanzapine; and produce weight gain similar to risperidone, clozapine and aripiprazole. They concluded that it produces suicide attempt, suicide; death; QTc prolongation, low blood pressure; tachycardia; sedation; gynaecomastia; galactorrhoea, menstrual irregularity and white blood cell count at a rate similar to first generation antipsychotics.[20]
الاضطراب ثنائي القطب
In those with bipolar disorder, quetiapine is used to treat depressive episodes; acute manic episodes associated with bipolar I disorder (as either monotherapy or adjunct therapy to lithium; valproate or lamotrigine); and maintenance treatment of bipolar I disorder (as adjunct therapy to lithium or divalproex).
الاكتئاب الحاد
Quetiapine is effective when used by itself[7] and when used along with other medications in major depressive disorder (MDD).[7][21] However, sedation is often an undesirable side effect.[7]
In the United States,[3] the United Kingdom[22] and Australia (while not subsidised by the Australian Pharmaceutical Benefits Scheme for treatment of MDD), quetiapine is licensed for use as an add-on treatment in MDD.[23]
مرض ألزايمر
Quetiapine does not decrease agitation among people with Alzheimer's. Quetiapine worsens intellectual functioning in the elderly with dementia and therefore is not recommended.[24]
أخرى
The use of low doses of quetiapine for insomnia, while common, is not recommended; there is little evidence of benefit and concerns regarding adverse effects.[25][26]
It is sometimes used off-label, often as an augmentation agent, to treat conditions such as Tourette syndrome,[27] musical hallucinations[28] and anxiety disorders.[29]
Quetiapine and clozapine are the most widely used medications for the treatment of Parkinson's disease psychosis due to their very low extrapyramidal side-effect liability. Owing to the risks associated with clozapine (e.g. agranulocytosis, diabetes mellitus, etc.), clinicians often attempt treatment with quetiapine first, although the evidence to support quetiapine's use for this indication is significantly weaker than that of clozapine.[30][31]
الآثار الجانبية
Sources for incidence lists:[1][3][22][23][31][32]
- Very common (>10% incidence) adverse effects
- Dry mouth
- Dizziness
- Headache
- Somnolence (drowsiness; of 15 antipsychotics quetiapine causes the 5th most sedation. Extended release (XR) formulations tend to produce less sedation, dose-by-dose than the immediate release formulations)[16]
- Common (1–10% incidence) adverse effects
- High blood pressure
- Orthostatic hypotension
- High pulse rate
- High blood cholesterol
- Elevated serum triglycerides
- Abdominal pain
- Constipation
- Increased appetite
- Vomiting
- Increased liver enzymes
- Backache
- Asthenia
- Insomnia
- Lethargy
- Tremor
- Agitation
- Nasal congestion
- Pharyngitis
- Fatigue
- Pain
- Dyspepsia (Indigestion)
- Peripheral oedema
- Dysphagia
- Extrapyramidal disease: quetiapine and clozapine are noted for their relative lack of extrapyramidal side effects[16][22][31]
- Weight gain: SMD 0.43 kg when compared to placebo. Produces roughly as much weight gain as risperidone, less weight gain than clozapine, olanzapine and zotepine and more weight gain than ziprasidone, lurasidone, aripiprazole and asenapine.[16] As with many other atypical antipsychotics, this action is likely due to its actions at the H1 histamine receptor and 5-HT2C receptor.[5]
- Rare (<1% incidence) adverse effects
- Prolonged QT interval (had an odds ratio for prolonging the QT interval over placebo of 0.17)[16]
- Sudden cardiac death
- Syncope
- Diabetic ketoacidosis
- Restless legs syndrome
- Hyponatraemia, low blood sodium.
- Jaundice, yellowing of the eyes, skin and mucous membranes due to an impaired ability of the body to clear bilirubin, a by product of haem breakdown.
- Pancreatitis, pancreas swelling.
- Agranulocytosis, a potentially fatal drop in white blood cell count.
- Leukopenia, a drop in white blood cell count, not as severe as agranulocytosis.
- Neutropenia, a drop in neutrophils, the cell of the immune cells that defends the body against bacterial infections.
- Eosinophilia
- Anaphylaxis, a potentially fatal allergic reaction.
- Seizure
- Hypothyroidism, underactive thyroid gland.
- Myocarditis, swelling of the myocardium.
- Cardiomyopathy
- Hepatitis, swelling of the liver.
- Suicidal ideation
- Priapism. A prolonged and painful erection.
- Stevens-Johnson syndrome. A potentially fatal skin reaction.
- Neuroleptic malignant syndrome a rare and potentially fatal complication of antipsychotic drug treatment. It is characterised by the following symptoms: tremor, rigidity, hyperthermia, tachycardia, mental status changes (e.g. confusion), etc.
- Tardive Dyskinesia. A rare and often irreversible neurological condition characterised by involuntary movements of the face, tongue, lips and rest of the body. Most commonly occurs after prolonged treatment with antipsychotics. It is believed to be particularly uncommon with atypical antipsychotics, especially quetiapine and clozapine[23][33]
Both typical and atypical antipsychotics can cause tardive dyskinesia.[34] According to one study, rates are lower with the atypicals at 3.9% as opposed to the typicals at 5.5%.[34] Although quetiapine and clozapine are atypical antipsychotics, switching to these atypicals is an option to minimize symptoms of tardive dyskinesia caused by other atypicals.[35]
Weight gain can be a problem for some, with quetiapine causing more weight gain than fluphenazine, haloperidol, loxapine, molindone, olanzapine, pimozide, risperidone, thioridazine, thiothixene, trifluoperazine, and ziprasidone, but less than chlorpromazine, clozapine, perphenazine, and sertindole.[36]
As with some other anti-psychotics, quetiapine may lower the seizure threshold,[37] and should be taken with caution in combination with drugs such as bupropion.
التوقف عن الاستخدام
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[38] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[39] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[39] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[39] Symptoms generally resolve after a short period of time.[39]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[40] It may also result in reoccurrence of the condition that is being treated.[41] Rarely tardive dyskinesia can occur when the medication is stopped.[39]
الحمل والرضاة
Placental exposure is least for quetiapine compared to other atypical antipsychotics.[31] The evidence is insufficient to rule out any risk to the foetus but available data suggests it is unlikely to result in any major foetal malformations.[2][4][32] It is secreted in breast milk and hence quetiapine-treated mothers are advised not to breastfeed.[2][4][32]
الجرعة الزائدة
Most instances of acute overdosage result in only sedation, hypotension and tachycardia, but cardiac arrhythmia, coma and death have occurred in adults. Serum or plasma quetiapine concentrations are usually in the 1–10 mg/L range in overdose survivors, while postmortem blood levels of 10–25 mg/L are generally observed in fatal cases.[42] Non-toxic levels in postmortem blood extend to around 0.8 mg/kg, but toxic levels in postmortem blood can begin at 0.35 mg/kg.[43][44]
علم الأدوية
الحركة الدوائية
| Site | QTP | NQTP | Action | Ref |
|---|---|---|---|---|
| SERT | >10,000 | 927 | Blocker | [46] |
| NET | >10,000 | 58 | Blocker | [46] |
| DAT | >10,000 | >10,000 | ND | [46] |
| 5-HT1A | 320–432 | 45 | Partial agonist | [46][47] |
| 5-HT1B | 1,109–2,050 | 1,117 | ND | [46][47] |
| 5-HT1D | >10,000 | 249 | ND | [46][47] |
| 5-HT1E | 1,250–2,402 | 97 | ND | [46][47] |
| 5-HT1F | 2,240 | ND | ND | [47] |
| 5-HT2A | 96–101 | 48 | Antagonist | [46][47] |
| 5-HT2B | ND | 14 | Antagonist | [46] |
| 5-HT2C | 2,502 | 107 | Antagonist | [46] |
| 5-HT3 | >10,000 | 394 | Antagonist | [46] |
| 5-HT4 | ND | ND | ND | ND |
| 5-HT5A | 3,120 | 768 | ND | [46] |
| 5-HT6 | 1,865 | 503 | Antagonist | [46] |
| 5-HT7 | 307 | 76 | Antagonist | [46] |
| α1A | 22 | 144 | Antagonist | [46] |
| α1B | 39 | 95 | Antagonist | [46] |
| α2A | 2,230–3,630 | 237 | Antagonist | [46][47] |
| α2B | 90–747 | 378 | Antagonist | [46][47] |
| α2C | 28.7–350 | 736 | Antagonist | [46][47] |
| β1 | >10,000 | >10,000 | ND | [46][47] |
| β2 | >10,000 | >10,000 | ND | [46][47] |
| D1 | 712 | 214 | Antagonist | [46] |
| D2 | 245 | 196 | Antagonist | [46] |
| D2L | 700 | ND | Antagonist | [47] |
| D2S | 390 | ND | Antagonist | [47] |
| D3 | 340–483 | 567 | Antagonist | [46][47] |
| D4 | 1,202 | 1,297 | Antagonist | [46] |
| D4.2 | 1,600 | ND | Antagonist | [47] |
| D5 | 1,738 | 1,419 | Antagonist | [46] |
| H1 | 2.2–11 | 3.5 | Antagonist | [46][47] |
| H2 | >10,000 | 298 | Antagonist | [46] |
| H3 | >10,000 | >10,000 | ND | [46] |
| H4 | >10,000 | 1,660 | ND | [46] |
| M1 | 858 | 39 | Antagonist | [46] |
| M2 | 1,339 | 453 | ND | [46] |
| M3 | >10,000 | 23 | Antagonist | [46] |
| M4 | 542 | 110 | ND | [46] |
| M5 | 1,942 | 23 | Antagonist | [46] |
| σ1 | 220–3,651 | >10,000 | ND | [46][47] |
| σ2 | 1,344 | 1,050 | ND | [46] |
| NMDA (PCP) |
>10,000 | ND | Antagonist | [46] |
| VDCC | >10,000 | ND | ND | [46][47] |
| hERG | ND | >10,000 (IC50) |
ND | [46] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. All data are for human cloned proteins, except σ1 (guinea pig), σ2 (rat), and VDCC (rat).[46][47] | ||||
Quetiapine has the following pharmacological actions:[48][49][50][51][52][53][54][55]
- Dopamine D1, D2, D3, D4, and D5 receptor antagonist
- Serotonin 5-HT1A receptor partial agonist, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7 receptor antagonist, and 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptor ligand
- α1- and α2-adrenergic receptor antagonist
- Histamine H1 receptor antagonist
- Muscarinic acetylcholine receptor antagonist
This means quetiapine is a dopamine, serotonin, and adrenergic antagonist, and a potent antihistamine with some anticholinergic properties.[56] Quetiapine binds strongly to serotonin receptors; the drug acts as partial agonist at 5-HT1A receptors.[57] Serial PET scans evaluating the D2 receptor occupancy of quetiapine have demonstrated that quetiapine very rapidly disassociates from the D2 receptor.[58] Theoretically, this allows for normal physiological surges of dopamine to elicit normal effects in areas such as the nigrostriatal and tuberoinfundibular pathways, thus minimizing the risk of side-effects such as pseudo-parkinsonism as well as elevations in prolactin.[59] Some of the antagonized receptors (serotonin, norepinephrine) are actually autoreceptors whose blockade tends to increase the release of neurotransmitters.
At very low doses, quetiapine acts primarily as a histamine receptor blocker (antihistamine) and α1-adrenergic blocker. When the dose is increased, quetiapine activates the adrenergic system and binds strongly to serotonin receptors and autoreceptors. At high doses, quetiapine starts blocking significant amounts of dopamine receptors.[49][60] Off-label prescriptions, e.g. for chronic insomnia, of low-dose quetiapine is not recommended due to the harmful side-effects.[61]
When treating schizophrenia, antagonism of D2 receptor by quetiapine in the mesolimbic pathway relieves positive symptoms and antagonism of the 5HT2A receptor in the frontal cortex of the brain relieves negative symptoms.[14][62][63] Quetiapine has fewer extrapyramidal side effects and is less likely to cause hyperprolactinemia when compared to other drugs used to treat schizophrenia, so is used as a first line treatment.[64][65]
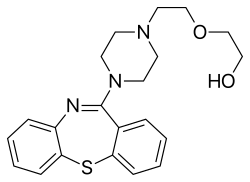
الكيمياء
Quetiapine is a tetracyclic compound and is closely related structurally to clozapine, olanzapine, loxapine, and other tetracyclic antipsychotics.
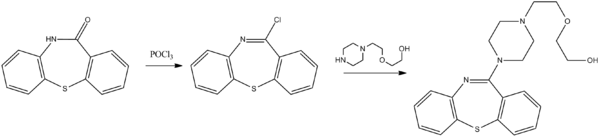
التحضير
The synthesis of quetiapine begins with a dibenzothiazepinone. The lactam is first treated with phosphoryl chloride to produce a dibenzothiazepine. A nucleophilic substitution is used to introduce the sidechain.[66]
التاريخ
المجتمع والثقافة
الوضع التنظيمي
التكلفة
In the United States as of 2015, the branded extended-release 400 mg pills cost between US$9.68 and US$23.16 each.[67] In 2017, the short-acting version had a wholesale cost of about US$12 per month.[11]
In the United Kingdom, a month's supply, as of 2017, costs the NHS approximately £107.45.[10] This is following an increase of 30 to 70 fold in 2017/2018.[68]
الدعاوى القضائية
In April 2010, the U. S. Department of Justice fined Astra-Zeneca $520 million for the company's aggressive marketing of Seroquel for off-label uses.[69] According to the Department of Justice, "the company recruited doctors to serve as authors of articles that were ghostwritten by medical literature companies and about studies the doctors in question did not conduct. AstraZeneca then used those studies and articles as the basis for promotional messages about unapproved uses of Seroquel."[69]
Multiple lawsuits have been filed in relation to quetiapine's side-effects, in particular, diabetes.[70][71][72][73]
Approximately 10,000[74] lawsuits[75] have been filed against AstraZeneca, alleging that quetiapine caused problems ranging from slurred speech and chronic insomnia to deaths.
جدل
In 2004, a young man named Dan Markingson committed suicide in a controversial Seroquel clinical trial at the University of Minnesota while under an involuntary commitment order.[76] A group of University of Minnesota bioethicists charged that the trial involved an alarming number of ethical violations.[77]
حالة تلاعب نيوروفن پلس
In August 2011, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) issued a class-4 drug alert following reports that some batches of Nurofen plus contained Seroquel XL instead.[78]
Following the issue of the Class-4 Drug Alert, Reckitt Benckiser (UK) Ltd received further reports of rogue blister strips in cartons of two additional batches of Nurofen Plus tablets. One of the new batches contained Seroquel XL 50 mg tablets and one contained the Pfizer product Neurontin 100 mg capsules.
Following discussions with the MHRA's Defective Medicines Report Centre (DMRC), Reckitt Benckiser (UK) Ltd decided to recall all remaining unexpired stock of Nurofen Plus tablets in any pack size, leading to a Class-1 Drug Alert.[79] The contamination was later traced to in-store tampering by a customer.[80]
المصادر
- ^ أ ب ت "quetiapine (Rx) - Seroquel, Seroquel XR". Medscape Reference. WebMD. Archived from the original on 20 October 2013. Retrieved 11 October 2013.
- ^ أ ب ت ث ج "Quetiapine 25 mg film-coated tablets - Summary of Product Characteristics". electronic Medicines Compendium. Sandoz. يناير 2013. Archived from the original on 20 أكتوبر 2013. Retrieved 20 أكتوبر 2013.
- ^ أ ب ت ث Truven Health Analytics, Inc. DrugPoint System (Internet) [cited 2013 Sep 18]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^ أ ب ت "QUETIAPINE FUMARATE tablet QUETIAPINE FUMARATE (quetiapine fumarate ) tablet [Ascend Laboratories, LLC]". DailyMed. Ascend Laboratories, LLC. October 2013. Archived from the original on 2 December 2013. Retrieved 26 November 2013.
- ^ أ ب Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill Professional. ISBN 978-0071624428.
- ^ أ ب ت ث ج ح خ د "Quetiapine Fumarate". The American Society of Health-System Pharmacists. Archived from the original on 29 August 2017. Retrieved 26 March 2017.
- ^ أ ب ت ث Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- ^ Anderson SL, Vande Griend JP (March 2014). "Quetiapine for insomnia: A review of the literature". American Journal of Health-System Pharmacy. 71 (5): 394–402. doi:10.2146/ajhp130221. PMID 24534594.
- ^ Riedel M, Müller N, Strassnig M, Spellmann I, Severus E, Möller HJ (April 2007). "Quetiapine in the treatment of schizophrenia and related disorders". Neuropsychiatric Disease and Treatment. 3 (2): 219–35. doi:10.2147/nedt.2007.3.2.219. PMC 2654633. PMID 19300555.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ أ ب ت British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. 383. ISBN 978-0857112989.
- ^ أ ب "NADAC as of 2017-03-22". Centers for Medicare and Medicaid Services. Archived from the original on 26 March 2017. Retrieved 26 March 2017.
- ^ "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- ^ "quetiapine-fumarate". The American Society of Health-System Pharmacists. Archived from the original on 10 February 2011. Retrieved 3 April 2011.
- ^ أ ب Dev V, Raniwalla J (October 2000). "Quetiapine: a review of its safety in the management of schizophrenia". Drug Safety. 23 (4): 295–307. doi:10.2165/00002018-200023040-00003. PMID 11051217.
- ^ أ ب Suttajit S, Srisurapanont M, Xia J, Suttajit S, Maneeton B, Maneeton N (May 2013). "Quetiapine versus typical antipsychotic medications for schizophrenia". The Cochrane Database of Systematic Reviews. 5 (5): CD007815. doi:10.1002/14651858.CD007815.pub2. PMID 23728667. Archived from the original on 2016-04-21.
- ^ أ ب ت ث ج Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- ^ أ ب Srisurapanont M, Maneeton B, Maneeton N (2004). Srisurapanont M (ed.). "Quetiapine for schizophrenia". The Cochrane Database of Systematic Reviews (2): CD000967. doi:10.1002/14651858.CD000967.pub2. PMID 15106155.
- ^ Kane JM, Correll CU (2010). "Pharmacologic treatment of schizophrenia". Dialogues Clin Neurosci. 12 (3): 345–57. PMC 3085113. PMID 20954430.
- ^ Schultz SH, North SW, Shields CG (June 2007). "Schizophrenia: a review". American Family Physician. 75 (12): 1821–9. PMID 17619525.
- ^ Asmal L, Flegar SJ, Wang J, Rummel-Kluge C, Komossa K, Leucht S (November 2013). "Quetiapine versus other atypical antipsychotics for schizophrenia". The Cochrane Database of Systematic Reviews. 11 (11): CD006625. doi:10.1002/14651858.CD006625.pub3. PMC 4167871. PMID 24249315.
- ^ Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (2013). "Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes". PLoS Medicine. 10 (3): e1001403. doi:10.1371/journal.pmed.1001403. PMC 3595214. PMID 23554581.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ أ ب ت British National Formulary (BNF) 65. London, UK: Pharmaceutical Press. 2013. p. 235. ISBN 9780857110848.
- ^ أ ب ت Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. (April 2005). "Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial". BMJ. 330 (7496): 874. doi:10.1136/bmj.38369.459988.8F. PMC 556156. PMID 15722369.
- ^ Coe HV, Hong IS (May 2012). "Safety of low doses of quetiapine when used for insomnia". The Annals of Pharmacotherapy. 46 (5): 718–22. doi:10.1345/aph.1Q697. PMID 22510671.
- ^ Maglione M, Maher AR, Hu J, Wang Z, Shanman R, Shekelle PG, Roth B, Hilton L, Suttorp MJ, Ewing BA, Motala A, Perry T (September 2011). "Off-Label Use of Atypical Antipsychotics: An Update". PMID 22132426.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Mukaddes NM, Abali O (2003). "Quetiapine treatment of children and adolescents with Tourette's disorder". Journal of Child and Adolescent Psychopharmacology. 13 (3): 295–9. doi:10.1089/104454603322572624. PMID 14642017.
- ^ Oliver Sacks "Musicophilia" Knopf NY 2007 P.67
- ^ Becker PM (September 2006). "Treatment of sleep dysfunction and psychiatric disorders". Current Treatment Options in Neurology. 8 (5): 367–75. doi:10.1007/s11940-006-0026-6. PMID 16901376.
- ^ Shotbolt P, Samuel M, David A (November 2010). "Quetiapine in the treatment of psychosis in Parkinson's disease". Therapeutic Advances in Neurological Disorders. 3 (6): 339–50. doi:10.1177/1756285610389656. PMC 3002640. PMID 21179595.
- ^ أ ب ت ث Taylor D, Carol P, Shitij K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 9780470979693.
- ^ أ ب ت "PRODUCT INFORMATION STADA(TM) Quetiapine (quetiapine fumarate Tablets 25 mg, 100 mg, 200 mg, 300 mg)". TGA eBusiness Services. STADA Pharmaceuticals Australia Pty Limited. 30 November 2012. Archived from the original on 27 March 2017. Retrieved 19 September 2013.
- ^ Erkan, A; Pirildar, S; Acarer, A; Akdeniz, F (2011). "Efficacy of Low-dose Pramipexole Augmentation in the Treatment of Refractory Psychotic Depression Complicated with Tardive Dyskinesia A Case Report" (PDF). Bulletin of Clinical Psychopharmacology (in Turkish). 21 (4): 353–355. doi:10.5455/bcp.20111029071711. Archived from the original (PDF) on 2012-01-13. Retrieved 2018-12-12.
{{cite journal}}: CS1 maint: unrecognized language (link) - ^ أ ب Correll CU, Schenk EM (March 2008). "Tardive dyskinesia and new antipsychotics". Current Opinion in Psychiatry. 21 (2): 151–6. doi:10.1097/YCO.0b013e3282f53132. PMID 18332662.
- ^ Aia PG, Revuelta GJ, Cloud LJ, Factor SA (June 2011). "Tardive dyskinesia". Current Treatment Options in Neurology. 13 (3): 231–41. doi:10.1007/s11940-011-0117-x. PMID 21365202.
- ^ Antipsychotic-Induced Weight Gain: A Comprehensive Research Synthesis Archived 2012-04-25 at the Wayback Machine Am J Psychiatry 1999;156:1686-1696.
- ^ Seroquel Prescribing Information
- ^ Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ^ أ ب ت ث ج Haddad, Peter; Haddad, Peter M.; Dursun, Serdar; Deakin, Bill (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide (in الإنجليزية). OUP Oxford. pp. 207–216. ISBN 9780198527480.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655.
- ^ Sacchetti, Emilio; Vita, Antonio; Siracusano, Alberto; Fleischhacker, Wolfgang (2013). Adherence to Antipsychotics in Schizophrenia (in الإنجليزية). Springer Science & Business Media. p. 85. ISBN 9788847026797.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1355–1357.
- ^ Skov L, Johansen SS, Linnet K (Jan 2015). "Postmortem femoral blood reference concentrations of aripiprazole, chlorprothixene, and quetiapine". Journal of Analytical Toxicology. 39 (1): 41–4. doi:10.1093/jat/bku121. PMID 25342720.
- ^ Skov L, Johansen SS, Linnet K (September 2015). "Postmortem Quetiapine Reference Concentrations in Brain and Blood". Journal of Analytical Toxicology. 39 (7): 557–61. doi:10.1093/jat/bkv072. PMID 26159868.
- ^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ أ ب ت ث ج ح خ د ذ ر ز س ش ص ض ط ظ ع غ ف ق ك ل م ن هـ و ي أأ أب أت أث أج أح أخ أد أذ أر أز أس أش أص Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL (September 2008). "N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity". Neuropsychopharmacology. 33 (10): 2303–12. doi:10.1038/sj.npp.1301646. PMID 18059438.
- ^ أ ب ت ث ج ح خ د ذ ر ز س ش ص ض ط ظ ع غ Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. (March 1996). "Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding". Psychopharmacology. 124 (1–2): 57–73. doi:10.1007/bf02245606. PMID 8935801.
- ^ AstraZeneca. "Seroquel (quietapine fumarate) tablets" (PDF). 276521. Archived from the original (PDF) on 2008-04-14.
{{cite journal}}: Cite journal requires|journal=(help) - ^ أ ب Richelson E, Souder T (November 2000). "Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds". Life Sciences. 68 (1): 29–39. doi:10.1016/S0024-3205(00)00911-5. PMID 11132243.
- ^ Davis, Kenneth L; Neuropsychopharmacology, American College of (2002). Neuropsychopharmacology: the fifth ... - Google Books. ISBN 978-0-7817-2837-9.
- ^ "Seroquel Official FDA information, side effects and uses". Drugs.com. Archived from the original on 2012-06-04. Retrieved 2012-07-09.
- ^ AstraZeneca Pharmaceuticals LP (March 2011). "SEROQUEL (quetiapine fumarate) tablet, extended release". DailyMed. National Library of Medicine. Section 12.2: Pharmacodynamics. Retrieved 2011-04-26.
- ^ National Institute of Mental Health. PDSD Ki Database (Internet) [cited 2013 Sep 18]. Chapel Hill (NC): University of North Carolina. 1998-2013. Available from: "Archived copy". Archived from the original on November 8, 2013. Retrieved July 5, 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL (September 2008). "N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity". Neuropsychopharmacology. 33 (10): 2303–12. doi:10.1038/sj.npp.1301646. PMID 18059438.
- ^ López-Muñoz F, Alamo C (September 2013). "Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders". Frontiers in Psychiatry. 4: 102. doi:10.3389/fpsyt.2013.00102. PMC 3770982. PMID 24062697.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, et al. (July 2008). "Anticholinergic activity of 107 medications commonly used by older adults". Journal of the American Geriatrics Society. 56 (7): 1333–41. doi:10.1111/j.1532-5415.2008.01737.x. PMID 18510583.
- ^ Guzman F. "Mechanism of action of quetiapine". Psychopharmacology Institute. Archived from the original on 11 August 2013. Retrieved 20 January 2013.
- ^ Kapur S, Seeman P (March 2001). "Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis". The American Journal of Psychiatry. 158 (3): 360–9. doi:10.1176/appi.ajp.158.3.360. PMID 11229973.
- ^ Seeman P (February 2002). "Atypical antipsychotics: mechanism of action". Canadian Journal of Psychiatry. 47 (1): 27–38. doi:10.1177/070674370204700106. PMID 11873706.
- ^ Gefvert O, Lundberg T, Wieselgren IM, Bergström M, Långström B, Wiesel F, Lindström L (April 2001). "D(2) and 5HT(2A) receptor occupancy of different doses of quetiapine in schizophrenia: a PET study". European Neuropsychopharmacology. 11 (2): 105–10. doi:10.1016/S0924-977X(00)00133-4. PMID 11313155.
- ^ "Drug Use Evaluation: Low Dose Quetiapine (Seroquel/Seroquel XR)" (PDF). Government Executive Media Group. Oregon State. Archived (PDF) from the original on 22 April 2016. Retrieved 20 January 2016.
- ^ "Quetiapine". www.drugbank.ca. Retrieved 23 January 2019.
- ^ "Seroquel" (PDF). FDA. Retrieved 23 January 2019.
- ^ Nemeroff CB, Kinkead B, Goldstein J (2002). "Quetiapine: preclinical studies, pharmacokinetics, drug interactions, and dosing". The Journal of Clinical Psychiatry. 63 Suppl 13: 5–11. PMID 12562141.
- ^ Kasper S, Müller-Spahn F (May 2000). "Review of quetiapine and its clinical applications in schizophrenia". Expert Opinion on Pharmacotherapy. 1 (4): 783–801. doi:10.1517/14656566.1.4.783. PMID 11249516.
- ^ Warawa, E. J.; Migler, B. M.; 1988, U.S. Patent 4٬879٬288.
- ^ Langreth, Robert (June 29, 2016). "Decoding Big Pharma's Secret Drug Pricing Practices". Bloomberg. Archived from the original on 13 July 2016. Retrieved 15 July 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "NHS hit by seven-fold increase in bill for certain drugs". Health Service Journal. 8 June 2018. Retrieved 29 August 2018.
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةOff-label - ^ Knef, Ann (2007-08-02). "Seroquel suit claims 'so much' is poured into marketing and away from research". The Madison / St. Clair Record. Archived from the original on 2008-08-21.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Milford, Phil (2009-03-11). "AstraZeneca May Link Seroquel, Diabetes, Doctor Says". Bloomberg.com. Bloomberg L.P. Archived from the original on 2015-09-24.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "AstraZeneca wins bellwether Seroquel case". FiercePharma. 2010-03-19. Archived from the original on 2012-03-14. Retrieved 2012-07-09.
- ^ "AstraZeneca pays out million dollar damages". The Local. 2010-08-09. Archived from the original on 2010-08-17.
- ^ "Questions loom over drug for sleepless vets - Marine Corps News | News from Afghanistan & Iraq". Marine Corps Times. Archived from the original on 2013-06-02. Retrieved 2012-07-09.
- ^ DUFF WILSON (2011-07-19). "Heart Warning Added to Label on Popular Antipsychotic Drug". NyTimes. Archived from the original on July 2, 2012. Retrieved 2012-07-09.
- ^ Elliott, Carl (September–October 2010). "The Deadly Corruption of Clinical Trials". Mother Jones. Archived from the original on 17 November 2012. Retrieved 2 December 2012.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Couzin-Frankel, Jennifer (December 7, 2010). "Minnesota bioethicists critique their university". Science. Archived from the original on 21 February 2013. Retrieved 2 December 2012.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Press releases". MHRA. Archived from the original on 2012-03-19. Retrieved 2012-07-09.
- ^ "Drug Alerts". MHRA. Archived from the original on 2012-03-19. Retrieved 2012-07-09.
- ^ "BBC News - Nurofen Plus tampering: Christopher McGuire jailed". Bbc.co.uk. 2012-05-28. Archived from the original on 2012-06-14. Retrieved 2012-07-09.
وصلات خارجية
- "Quetiapine". MedlinePlus. The American Society of Health-System Pharmacists, Inc. 2008-09-01.
- NAMI summary
- Internet Drug List summary
- Compound #1802: Quetiapine[dead link] ChemBank
- U.S. National Library of Medicine: Drug Information Portal - Quetiapine
- Australian Public Assessment Report for Quetiapine (as fumarate)
- CS1 maint: unflagged free DOI
- CS1 errors: unsupported parameter
- Template:drugs.com link with non-standard subpage
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Drugboxes which contain changes to watched fields
- Articles with dead external links from July 2016
- Alpha blockers
- مضادات اكتئاب
- Atypical antipsychotics
- AstraZeneca brands
- Dibenzothiazepines
- Ethers
- H1 receptor antagonists
- Hypnotics
- Mood stabilizers
- Piperazines
- كحولات أولية
- Sedatives
- RTT