پروپرانولول
 | |
 | |
| البيانات السريرية | |
|---|---|
| License data |
|
| فئة السلامة أثناء الحمل |
|
| مسارات الدواء | oral, iv |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | 26% |
| الأيض | hepatic (extensive) |
| Elimination half-life | 4-5 hours |
| الإخراج | renal <1% |
| المعرفات | |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.618 |
| Chemical and physical data | |
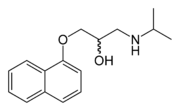
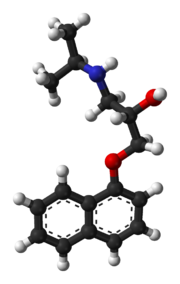
| التركيب | C16H21NO2 |
| الكتلة المولية | 259.34 g/mol |
پروپرانولول Propranolol (INN) يعد غير انتقائي beta blocker يستعمل غالبا لعلاج فرط ضغط الدم. يعتبر أول مثبط لمستقبلات بيتا يتم تطويره. ويعد أول دواء تثبت فعاليته للوقاية prophylaxisمن الصداع النصفى في الأطفال. بروبرانولول متوافر في شكل تجارى ك propranolol hydrochloride, بالإضافة الىAstraZeneca و Wyethمنتجاتإسم تجارى Inderal, Inderal LA, Avlocardyl (متوافر بصورة ممتدة المفعول لإمتصاصها البطىء تدعى "Avlocardyl Retard"), Deralin, Dociton, Inderalici, InnoPran XL, Sumial (تعتمد على مكان التسويق, ومعدل تحرر المادة الفعالة).
التاريخ والتطور
العالم الاسكتلندي خريج جامعة سانت أندروز، جيمس بلاك طور بنجاح بروبرانولول في نهاية 1950. وقد منح جائزة جائزة نوبل في الطب لهذا الإكتشاف في 1988. بروبرانولول تم إشتقاقه من مثبطات بيتا- الأدرينالية الأولى dichloroisoprenaline و pronethalol. التطوير للتركيب المفتاحى, الذى أنجز من ذلك الوقت فصاعدا لكل مثبطات البيتا , وهو إدخال, قنطرة oxymethylene داخل الهيكل arylethanolamine بروبرانولول,وهكذا إزدادت فعالية المركب زيادة كبيرة.وهذأيضاأظهر بوضوح singeliminated carcinogenicity التى وجدت مع بروبرانولول في نماذج حيوانية. أدوية جديدة أكثر إنتقائية من مثبطات البتا الأدرينالية, مثل nebivolol تستخدم الآن لعلاج ضغط الدم.
الفارماكولوچى
بروبرانولول يعد non-selective beta blocker , ويعنى ذلك أنه, يبطل تأثير أدرينالين في كلا β1- و β2- مستقبلات أدرينالين ولديه فعالية داخلية لمضاهاة العصب السمبتاوى (ISA) لكن لديه فعالية إستقرار قوية للغشاء (فقط عند تركيزات دم عالية, مثلا عند الجرعات العالية). فقط L-propranolol مثبط المستقبلات الأدرينالية قويا, بينما D-propranolol ليس كذلك. ولذلك كلاهما لديه تأثير مخدر موضعى (موضعى) .
Pharmacokinetics
Propranolol is rapidly and completely absorbed, with peak plasma levels achieved approximately 1–3 hours after ingestion. Co-administration with food appears to enhance bioavailability. Despite complete absorption, propranolol has a variable bioavailability due to extensive first-pass metabolism. Hepatic impairment will therefore increase its bioavailability. The main metabolite 4-hydroxypropranolol, with a longer half-life (5.2–7.5 hours) than the parent compound (3–4 hours), is also pharmacologically active.
Propranolol is a highly lipophilic drug achieving high concentrations in the brain. The duration of action of a single oral dose is longer than the half-life and may be up to 12 hours, if the single dose is high enough (e.g., 80 mg). Effective plasma concentrations are between 10–100 ng/mL.
Toxic levels are associated with plasma concentrations above 2000 ng/ml.
الاستعمال السريري
Indications
Propranolol is indicated for the management of various conditions including:[1]
- Hypertension
- Angina pectoris
- Tachyarrhythmias
- Myocardial infarction
- Control of tachycardia/tremor associated with anxiety, hyperthyroidism or lithium therapy.
- Essential tremor
- Migraine prophylaxis
- Cluster Headaches prophylaxis
- Tension headache (Off the label use)
- Tetralogy of Fallot
- Phaeochromocytoma (along with α blocker)
- There has been some experimentation in psychiatric areas:[2]
- Treating the excessive drinking of fluids in psychogenic polydipsia,[3][4]
- Antipsychotic-induced akathisia,[5]
- Aggressive behavior of patients with brain injuries[6]
- Post-traumatic stress disorder
- گلوكوما
While once first-line treatment for ارتفاع ضغط الدم, the role for beta-blockers was downgraded in June 2006 in the المملكة المتحدة to fourth-line as they perform less well than other drugs, particularly in the elderly, and evidence is increasing that the most frequently used beta-blockers at usual doses carry an unacceptable risk of provoking type 2 diabetes. [7]
پروپرانولول يـُستعمل أيضاً لخفض ضغط الوريد البابي في ارتفاع الضغط البابي ولمنع نزف دوالي المريء.
Propranolol is often used by musicians and other performers to prevent stage fright.
Propranolol is currently being investigated as a potential treatment for post-traumatic stress disorder. [8][9][10]
Precautions/contraindications
Propranolol should be used with caution in patients with:[1]
- Diabetes mellitus or hyperthyroidism, since signs and symptoms of hypoglycaemia may be masked. Also, propranolol may affect blood sugar levels
- Peripheral vascular disease and Raynaud's syndrome, which may be exacerbated
- Phaeochromocytoma, as hypertension may be aggravated without prior alpha blocker therapy
- Myasthenia gravis, may be worsened
- Other drugs with bradycardic effects
Propranolol is contraindicated in patients with:[1]
- Reversible airways disease, particularly asthma or chronic obstructive pulmonary disease (COPD)
- Bradycardia (<50 beats/minute)
- Sick sinus syndrome
- Atrioventricular block (second or third degree)
- Shock
- Severe hypotension
- Uncontrolled congestive heart failure
- Cocaine toxicity [per American Heart Association guidelines, 2005]
Adverse effects
Adverse drug reactions (ADRs) associated with propranolol therapy are similar to other lipophilic beta blockers (see beta blocker).
Pregnancy and lactation
Propranolol, like other beta blockers, is classified as Pregnancy category C in the United States and ADEC Category C in Australia. Beta-blocking agents in general reduce perfusion of the placenta which may lead to adverse outcomes for the neonate, including pulmonary or cardiac complications, or premature birth. The newborn may experience additional adverse effects such as hypoglycemia and bradycardia.
Most beta-blocking agents appear in the milk of lactating women. This is especially the case for a lipophilic drug like propranolol. Breastfeeding is not recommended in patients receiving propranolol therapy.
Interactions
Beta blockers, including propranolol, have an additive effect with other drugs which decrease blood pressure, or which decrease cardiac contractility or conductivity. Clinically-significant interactions particularly occur with:[1]
- verapamil
- epinephrine
- β2-adrenergic receptor agonists
- clonidine
- ergot alkaloids
- isoprenaline
- non-steroidal anti-inflammatory drugs
- quinidine
- cimetidine
- lidocaine
- phenobarbital
- rifampicin
- Fluvoxamine slows down the metabolism of propranolol significantly leading to increased blood levels of propranolol.[11]
Dosage
The usual maintenance dose ranges for oral propranolol therapy vary by indication:
- Hypertension, angina, essential tremor
- 120–320 mg daily in divided doses
- Sustained-release formulations are available in some markets.
- Tachyarrhythmia, anxiety (GAD), hyperthyroidism
- 10–40 mg 3–4 times daily
- Performance anxiety
- 5-10 mg 30min or 1.5hrs before and after performance, optionally 5-10 mg night before. Up to 40mg if necessary, but side-effects may present. [12]
Intravenous (IV) propranolol may be used in acute arrhythmia or thyrotoxic crisis.[13]
Research into role against malaria
Propranolol along with a number of other membrane-acting drugs have been investigated for possible effects on P. falciparum and so the treatment of malaria. In vitro positive effects until recently had not been matched by useful in vivo anti-parasite activity against P. vinckei,[14] or P. yoelii nigeriensis.[15] However a single study from 2006 has suggested that propranolol may reduce the dosages required for existing drugs to be effective against P. falciparum by 5- to 10-fold, suggesting a role for combination therapies.[16]
References
- ^ أ ب ت ث Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ^ Kornischka J, Cordes J, Agelink MW (2007). "[40 years beta-adrenoceptor blockers in psychiatry]". Fortschr Neurol Psychiatr (in German). 75 (4): 199–210. doi:10.1055/s-2006-944295. PMID 17200914.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unrecognized language (link) - ^ Vieweg V, Pandurangi A, Levenson J, Silverman J (1994). "The consulting psychiatrist and the polydipsia-hyponatremia syndrome in schizophrenia". Int J Psychiatry Med. 24 (4): 275–303. PMID 7737786.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kishi Y, Kurosawa H, Endo S (1998). "Is propranolol effective in primary polydipsia?". Int J Psychiatry Med. 28 (3): 315–25. PMID 9844835.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kramer MS, Gorkin R, DiJohnson C (1989). "Treatment of neuroleptic-induced akathisia with propranolol: a controlled replication study". Hillside J Clin Psychiatry. 11 (2): 107–19. PMID 2577308.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thibaut F, Colonna L (1993). "[Anti-aggressive effect of beta-blockers]". Encephale (in French). 19 (3): 263–7. PMID 7903928.
{{cite journal}}: CS1 maint: unrecognized language (link) - ^ Sheetal Ladva (2006-06-28). "NICE and BHS launch updated hypertension guideline". National Institute for Health and Clinical Excellence. Retrieved 2006-09-30.
- ^ "Doctors test a drug to ease traumatic memories - Mental Health - MSNBC.com". Retrieved 2007-06-30.
- ^ Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK (2007). "Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder": 503. doi:10.1016/j.jpsychires.2007.05.006. PMID 17588604.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ "Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder".
- ^ van Harten J (1995). "Overview of the pharmacokinetics of fluvoxamine". Clin Pharmacokinet. 29 Suppl 1: 1–9. PMID 8846617.
- ^ http://www.ncbi.nlm.nih.gov/pubmed/1686251?ordinalpos=16&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
- ^ *Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004.
- ^ Ohnishi S, Sadanaga K, Katsuoka M, Weidanz W (1990). "Effects of membrane acting-drugs on plasmodium species and sickle cell erythrocytes". Mol Cell Biochem. 91 (1–2): 159–65. doi:10.1007/BF00228091. PMID 2695829.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh N, Puri S (2000). "Interaction between chloroquine and diverse pharmacological agents in chloroquine resistant Plasmodium yoelii nigeriensis". Acta Trop. 77 (2): 185–93. doi:10.1016/S0001-706X(00)00133-9. PMID 11080509.
- ^ Murphy S, Harrison T, Hamm H, Lomasney J, Mohandas N, Haldar K (2006). "Erythrocyte G protein as a novel target for malarial chemotherapy". PLoS Med. 3 (12): e528. doi:10.1371/journal.pmed.0030528. PMID 17194200.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
External links
- CS1 errors: unsupported parameter
- CS1 maint: unflagged free DOI
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Articles without EBI source
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Antiarrhythmic agents
- Anxiolytics
- حاصرات بيتا
- اختراعات اسكتلندية