فلوريد الليثيوم

| |
 | |
 | |
| الأسماء | |
|---|---|
| اسم أيوپاك
فلوريد الليثيوم
| |
| المُعرِّفات | |
| رقم CAS | |
| ECHA InfoCard | 100.029.229 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
CompTox Dashboard (EPA)
|
|
| الخصائص | |
| الصيغة الجزيئية | LiF |
| كتلة مولية | 25.939(2) g/mol |
| المظهر | مسحوق أبيض أو شفاف بلوري non-hygroscopic |
| الكثافة | 2.635 g/cm3 |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 0.27 g/100 mL (18 °C) [1] |
| قابلية الذوبان | soluble in HF insoluble in alcohol |
| معامل الانكسار (nD) | 1.39937 |
| البنية | |
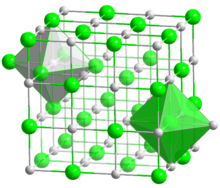
| البنية البلورية | Cubic |
| الشكل الجزيئي | Linear |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
-23.75 kJ/g |
| Standard molar entropy S |
1.376 J/(g K) |
| سعة الحرارة النوعية، C | 1.604 J/(g K) |
| مركبات ذا علاقة | |
أنيونات أخرى
|
كلوريد الليثيوم بروميد الليثيوم يوديد الليثيوم |
كاتيونات أخرى
|
فلوريد الصوديوم فلوريد البوتاسيوم فلوريد الروبيديوم فلوريد السيزيوم |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
فلوريد الليثيوم، هو مركب كيميائي من الليثيوم والفلورين. وهو ملح عضوي صلب بلوري أبيض اللون تحت الظروف القياسية.
Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts.[2] Partly because Li and F are both light elements, and partly because F
2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.
التصنيع
LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride.[3]
التطبيقات
سابق لسداسي فلوروفوسفات الليثيوم للبطاريات
Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make سداسي فلوروفوسفات الليثيوم Li[PF
6], an ingredient in lithium ion battery electrolyte.
The lithium fluoride alone does not absorb hydrogen fluoride to form a bifluoride salt.[4]
في الأملاح الذائبة
Fluorine is produced by the electrolysis of molten potassium bifluoride. This electrolysis proceeds more efficiently when the electrolyte contains a few percent of LiF, possibly because it facilitates formation of an Li-C-F interface on the carbon electrodes.[2] A useful molten salt, FLiNaK, consists of a mixture of LiF, together with sodium fluoride and potassium fluoride. The primary coolant for the Molten-Salt Reactor Experiment was FLiBe; 2LiF·BeF
2 (66 mol% of LiF, 33 mol% of BeF
2).
البصريات
Because of the large band gap for LiF, its crystals are transparent to short wavelength ultraviolet radiation, more so than any other material. LiF is therefore used in specialized optics for the vacuum ultraviolet spectrum.[5] (See also magnesium fluoride.) Lithium fluoride is used also as a diffracting crystal in X-ray spectrometry.
كشافات الإشعاع
It is also used as a means to record ionizing radiation exposure from gamma rays, beta particles, and neutrons (indirectly, using the 63Li (n,alpha) nuclear reaction) in thermoluminescent dosimeters. 6LiF nanopowder enriched to 96% has been used as the neutron reactive backfill material for microstructured semiconductor neutron detectors (MSND).[6]
المفاعلات النووية
Lithium fluoride (highly enriched in the common isotope lithium-7) forms the basic constituent of the preferred fluoride salt mixture used in liquid-fluoride nuclear reactors. Typically lithium fluoride is mixed with beryllium fluoride to form a base solvent (FLiBe), into which fluorides of uranium and thorium are introduced. Lithium fluoride is exceptionally chemically stable and LiF/BeF
2 mixtures (FLiBe) have low melting points (360 to 459 °C or 680 to 858 °F) and the best neutronic properties of fluoride salt combinations appropriate for reactor use. MSRE used two different mixtures in the two cooling circuits.
مهبط لكل من PLED و OLEDs
Lithium fluoride is widely used in PLED and OLED as a coupling layer to enhance electron injection. The thickness of the LiF layer is usually around 1 nm. The dielectric constant (or relative permittivity, ε) of LiF is 9.0.[7]
التواجد الطبيعي
Naturally occurring lithium fluoride is known as the extremely rare mineral griceite.[8]
المصادر
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ أ ب Aigueperse J, Mollard P, Devilliers D, et al. (2005). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 9783527303854.
- ^ Bellinger SL, Fronk RG, McNeil WJ, et al. (2012). "Improved High Efficiency Stacked Microstructured Neutron Detectors Backfilled With Nanoparticle 6LiF". IEEE Trans. Nucl. Sci. 59 (1): 167–173. Bibcode:2012ITNS...59..167B. doi:10.1109/TNS.2011.2175749. S2CID 19657691.
- ^ Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ "Lithium Fluoride (LiF) Optical Material". Crystran 19. 2012.
- ^ McGregor DS, Bellinger SL, Shultis JK (2013). "Present status of microstructured semiconductor neutron detectors". Journal of Crystal Growth. 379: 99–110. Bibcode:2013JCrGr.379...99M. doi:10.1016/j.jcrysgro.2012.10.061. hdl:2097/16983.
- ^ Andeen C, Fontanella J, Schuele D (1970). "Low-Frequency Dielectric Constant of LiF, NaF, NaCl, NaBr, KCl, and KBr by the Method of Substitution". Phys. Rev. B. 2 (12): 5068–73. Bibcode:1970PhRvB...2.5068A. doi:10.1103/PhysRevB.2.5068.
- ^ "Griceite mineral information and data". Mindat.org. Archived from the original on 7 March 2014. Retrieved 22 Jan 2014.
- "Lithium fluoride". Retrieved 2006-02-26.
خطأ لوا في وحدة:Authority_control على السطر 278: attempt to call field '_showMessage' (a nil value).