كلوريد الليثيوم
| |

| |
 | |
| الأسماء | |
|---|---|
| اسم أيوپاك المفضل
Lithium chloride | |
| اسم أيوپاك النظامي
Lithium(1+) chloride | |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.375 |
| رقم EC |
|
| عناوين مواضيع طبية MeSH | |
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
| UN number | 2056 |
CompTox Dashboard (EPA)
|
|
| الخصائص | |
| الصيغة الجزيئية | ClLi |
| كتلة مولية | 42.39 g mol-1 |
| المظهر | white solid hygroscopic, sharp |
| الكثافة | 2.068 g/cm3 |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 68.29 g/100 mL (0 °C) 74.48 g/100 mL (10 °C) 84.25 g/100 mL (25 °C) 88.7 g/100 mL (40 °C) 123.44 g/100 mL (100 °C)[1] |
| قابلية الذوبان | soluble in hydrazine, methylformamide, butanol, selenium(IV) oxychloride, 1-propanol[1] |
| قابلية الذوبان في methanol | 45.2 g/100 g (0 °C) 43.8 g/100 g (20 °C) 42.36 g/100 g (25 °C)[2] 44.6 g/100 g (60 °C)[1] |
| قابلية الذوبان في ethanol | 14.42 g/100 g (0 °C) 24.28 g/100 g (20 °C) 25.1 g/100 g (30 °C) 23.46 g/100 g (60 °C)[2] |
| قابلية الذوبان في formic acid | 26.6 g/100 g (18 °C) 27.5 g/100 g (25 °C)[1] |
| قابلية الذوبان في acetone | 1.2 g/100 g (20 °C) 0.83 g/100 g (25 °C) 0.61 g/100 g (50 °C)[1] |
| قابلية الذوبان في liquid ammonia | 0.54 g/100 g (-34 °C)[1] 3.02 g/100 g (25 °C) |
| ضغط البخار | 1 torr (785 °C) 10 torr (934 °C) 100 torr (1130 °C)[1] |
| القابلية المغناطيسية | −24.3·10−6 cm3/mol |
| معامل الانكسار (nD) | 1.662 (24 °C) |
| اللزوجة | 0.87 cP (807 °C)[1] |
| البنية | |
| هندسة إحداثية |
Octahedral |
| الشكل الجزيئي | Linear (gas) |
| Dipole moment | 7.13 D (gas) |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
-408.27 kJ/mol[1] |
| Standard molar entropy S |
59.31 J/mol·K[1] |
| سعة الحرارة النوعية، C | 48.03 J/mol·K[1] |
| علم الأدوية | |
| V04CX11 (WHO) | |
| المخاطر | |
| صفحة بيانات السلامة | ICSC 0711 |
| ن.م.ع. مخطط تصويري |  [3] [3]
|
| ن.م.ع. كلمة الاشارة | Warning |
| H302, H315, H319, H335[3] | |
| P261, P305+P351+P338[3] | |
| NFPA 704 (معيـَّن النار) | |
| نقطة الوميض | Non-flammable |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
526 mg/kg (oral, rat)[4] |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Lithium fluoride Lithium bromide Lithium iodide Lithium astatide |
كاتيونات أخرى
|
Sodium chloride Potassium chloride Rubidium chloride Caesium chloride Francium chloride |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties.[5]
الخصائص الكيميائية
The salt forms crystalline hydrates, unlike the other alkali metal chlorides.[6] Mono-, tri-, and pentahydrates are known.[7] The anhydrous salt can be regenerated by heating the hydrates. LiCl also absorbs up to four equivalents of ammonia/mol. As with any other ionic chloride, solutions of lithium chloride can serve as a source of chloride ion, e.g., forming a precipitate upon treatment with نترات الفضة:
- LiCl + AgNO3 → AgCl + LiNO3
Preparation
Lithium chloride is produced by treatment of lithium carbonate with hydrochloric acid.[5] Anhydrous LiCl is prepared from the hydrate by heating in a stream of hydrogen chloride.
Uses
Commercial applications
Lithium chloride is mainly used for the production of lithium metal by electrolysis of a LiCl/KCl melt at 450 °C (842 °F). LiCl is also used as a brazing flux for aluminium in automobile parts. It is used as a desiccant for drying air streams.[5] In more specialized applications, lithium chloride finds some use in organic synthesis, e.g., as an additive in the Stille reaction. Also, in biochemical applications, it can be used to precipitate RNA from cellular extracts.[8]
Lithium chloride is also used as a flame colorant to produce dark red flames.
Niche uses
Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. At 25 °C (77 °F) a saturated solution (45.8%) of the salt will yield an equilibrium relative humidity of 11.30%. Additionally, lithium chloride can be used as a hygrometer. This deliquescent salt forms a self-solution when exposed to air. The equilibrium LiCl concentration in the resulting solution is directly related to the relative humidity of the air. The percent relative humidity at 25 °C (77 °F) can be estimated, with minimal error in the range 10–30 °C (50–86 °F), from the following first-order equation: RH=107.93-2.11C, where C is solution LiCl concentration, percent by mass.
Molten LiCl is used for the preparation of carbon nanotubes,[9] graphene[10] and lithium niobate.[11]
Lithium chloride has been shown to have strong acaricidal properties, being effective against Varroa destructor in populations of honey bees.[12]
Lithium chloride is used as an aversive agent in lab animals to study conditioned place preference and aversion.
Precautions
Lithium salts affect the central nervous system in a variety of ways. While the citrate, carbonate, and orotate salts are currently used to treat bipolar disorder, other lithium salts including the chloride were used in the past. For a short time in the 1940s lithium chloride was manufactured as a salt substitute for people with hypertension, but this was prohibited after the toxic effects of the compound (tremors, fatigue, nausea) were recognized.[13][14][15] It was, however, noted by J. H. Talbott that many symptoms attributed to lithium chloride toxicity may have also been attributable to sodium chloride deficiency, to the diuretics often administered to patients who were given lithium chloride, or to the patients' underlying conditions.[13]
See also
References
- ^ أ ب ت ث ج ح خ د ذ ر ز lithium chloride
- ^ أ ب Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. Retrieved 2014-06-02.
- ^ أ ب ت Sigma-Aldrich Co., Lithium chloride. Retrieved on 2014-05-09.
- ^ ChemIDplus - 7447-41-8 - KWGKDLIKAYFUFQ-UHFFFAOYSA-M - Lithium chloride - Similar structures search, synonyms, formulas, resource links, and other chemical information
- ^ أ ب ت Wietelmann, Ulrich; Bauer, Richard J. (2005). "Lithium and Lithium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_393.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Hönnerscheid Andreas; Nuss Jürgen; Mühle Claus; Jansen Martin (2003). "Die Kristallstrukturen der Monohydrate von Lithiumchlorid und Lithiumbromid". Zeitschrift für anorganische und allgemeine Chemie. 629 (2): 312–316. doi:10.1002/zaac.200390049.
- ^ Cathala, G.; Savouret, J.; Mendez, B.; West, B. L.; Karin, M.; Martial, J. A.; Baxter, J. D. (1983). "A Method for Isolation of Intact, Translationally Active Ribonucleic Acid". DNA. 2 (4): 329–335. doi:10.1089/dna.1983.2.329. PMID 6198133.
- ^ Kamali, Ali Reza; Fray, Derek J. (2014). "Towards large scale preparation of carbon nanostructures in molten LiCl". Carbon. 77: 835–845. doi:10.1016/j.carbon.2014.05.089.
- ^ Kamali, Ali Reza; Fray, Derek J. (2015). "Large-scale preparation of graphene by high temperature insertion of hydrogen into graphite" (PDF). Nanoscale. 7 (26): 11310–11320. doi:10.1039/c5nr01132a. PMID 26053881.
- ^ Kamali, Ali Reza; Fray, Derek J. (2014). "Preparation of lithium niobate particles via reactive molten salt synthesis method". Ceramics International. 40: 1835–1841. doi:10.1016/j.ceramint.2013.07.085.
- ^ Ziegelmann, Bettina; Abele, Elisabeth (January 12, 2018). "Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action". Scientific Reports. 8 (1): 683. Bibcode:2018NatSR...8..683Z. doi:10.1038/s41598-017-19137-5. PMC 5766531. PMID 29330449.
- ^ أ ب Talbott J. H. (1950). "Use of lithium salts as a substitute for sodium chloride". Arch Intern Med. 85 (1): 1–10. doi:10.1001/archinte.1950.00230070023001. PMID 15398859.
- ^ L. J. Stone; M. luton; J. Gilroy (1949). "Lithium Chloride as a Substitute for Sodium Chloride in the Diet". Journal of the American Medical Association. 139 (11): 688–692. doi:10.1001/jama.1949.02900280004002. PMID 18128981.
- ^ "Case of trie Substitute Salt". Time. 28 February 1949. Archived from the original on March 2, 2007.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- R. Vatassery, titration analysis of LiCl, sat'd in Ethanol by AgNO3 to precipitate AgCl(s). EP of this titration gives %Cl by mass.
- H. Nechamkin, The Chemistry of the Elements, McGraw-Hill, New York, 1968.
External links
خطأ لوا في وحدة:Authority_control على السطر 278: attempt to call field '_showMessage' (a nil value).
- Short description is different from Wikidata
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Chlorides
- Alkali metal chlorides
- Lithium salts
- Metal halides
- Mood stabilizers
- Desiccants
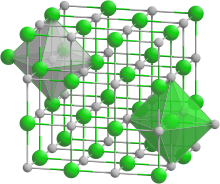
- Rock salt crystal structure

