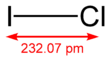أحادي كلوريد اليود
|
| |||

| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك المفضل
Iodine monochloride Iodine(I) chloride | |||
| اسم أيوپاك النظامي
Chloroiodane | |||
| أسماء أخرى
Iodine chloride
| |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.306 | ||
| رقم EC |
| ||
| عناوين مواضيع طبية MeSH | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1792 | ||
CompTox Dashboard (EPA)
|
|||
| الخصائص | |||
| الصيغة الجزيئية | ICl | ||
| كتلة مولية | 162.35 g/mol | ||
| المظهر | reddish-brown | ||
| الكثافة | 3.10 g/cm3 | ||
| نقطة الانصهار | |||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | Hydrolyzes | ||
| قابلية الذوبان | soluble in CS2 acetic acid pyridine alcohol, ether, HCl | ||
| القابلية المغناطيسية | −54.6×10−6 cm3/mol | ||
| المخاطر | |||
| خطر رئيسي | Corrosive, reacts with water to release HCl | ||
| صفحة بيانات السلامة | https://chemicalsafety.com/sds1/sdsviewer.php?id=30683304 | ||
| مركبات ذا علاقة | |||
interhalogen compounds ذات العلاقة
|
Chlorine monofluoride Bromine monochloride Iodine monobromide | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Discovered in 1814 by Gay-Lussac, iodine monochloride is the first interhalogen compound discovered.[1]
Preparation
Iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation
- I
2 + Cl
2 → 2 ICl
When chlorine gas is passed through iodine crystals, one observes the brown vapor of iodine monochloride. Dark brown iodine monochloride liquid is collected. Excess chlorine converts iodine monochloride into iodine trichloride in a reversible reaction:
- ICl + Cl
2 ⇌ ICl
3
Polymorphs
ICl has two polymorphs; α-ICl, which exists as black needles (red by transmitted light) with a melting point of 27.2 °C, and β-ICl, which exists as black platelets (red-brown by transmitted light) with a melting point 13.9 °C.[2]
In the crystal structures of both polymorphs the molecules are arranged in zigzag chains. β-ICl is monoclinic with the space group P21/c.[3]
Reactions and uses
Iodine monochloride is soluble in acids such as HF and HCl but reacts with pure water to form HCl, iodine, and iodic acid:
- ICl + H
2O → HCl + HI + 1⁄2O2 - 2 ICl + H
2O → 2 HCl + I
2 + 1⁄2O2 - 5 ICl + 3 H
2O → 5 HCl + HIO
3 + 2 I
2
ICl is a useful reagent in organic synthesis.[2] It is used as a source of electrophilic iodine in the synthesis of certain aromatic iodides.[4] It also cleaves C–Si bonds.
ICl will also add to the double bond in alkenes to give chloro-iodo alkanes.
- RCH=CHR′ + ICl → RCH(I)–CH(Cl)R′
When such reactions are conducted in the presence of sodium azide, the iodo-azide RCH(I)–CH(N3)R′ is obtained.[5]
The Wijs solution, iodine monochloride dissolved in acetic acid, is used to determine the iodine value of a substance.
It can also be used to prepare iodates, by reaction with a chlorate. Chlorine is released as a byproduct.
Iodine monochloride is a Lewis acid that forms 1:1 adducts with Lewis bases such as dimethylacetamide and benzene.
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 790. ISBN 978-0-08-037941-8.
- ^ أ ب Brisbois, R. G.; Wanke, R. A.; Stubbs, K. A.; Stick, R. V. "Iodine Monochloride" Encyclopedia of Reagents for Organic Synthesis, 2004 John Wiley & Sons. DOI:10.1002/047084289X.ri014
- ^ Carpenter, G. B.; Richards, S. M. (1 April 1962). "The crystal structure of β-iodine monochloride". Acta Crystallographica. 15 (4): 360–364. doi:10.1107/S0365110X62000882.
- ^ Wallingford, V. H.; Krüger, P. A. (1943). "5-Iodo-anthranilic Acid". Organic Syntheses; Collected Volumes, 2, pp. 349.
- ^ Padwa, A.; Blacklock, T.; Tremper, A. "3-Phenyl-2H-Azirine-2-carboxaldehyde". Organic Syntheses; Collected Volumes, 6, pp. 893.