حديدوسيانيد الپوتاسيوم
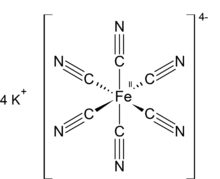
| |
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Potassium hexacyanidoferrate(II)
| |
| أسماء أخرى | |
| المُعرِّفات | |
| رقم CAS | |
| ECHA InfoCard | 100.034.279 |
| E number | E536 (acidity regulators, ...) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| الخصائص | |
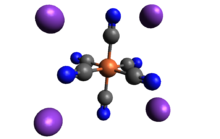
| الصيغة الجزيئية | K4[Fe(CN)6] |
| كتلة مولية | 368.35 g/mol (anhydrous) 422.388 g/mol (trihydrate) |
| المظهر | Light yellow, crystalline granules |
| الكثافة | 1.85 g/cm3 (trihydrate) |
| قابلية الذوبان في الماء | trihydrate 28.9 g/100 mL (20 °C) |
| قابلية الذوبان | insoluble in ethanol, ether |
| القابلية المغناطيسية | −130.0·10−6 cm3/mol |
| المخاطر | |
| توصيف المخاطر | R32, R52, R53 |
| تحذيرات وقائية | قالب:S50(B), S61 |
| NFPA 704 (معيـَّن النار) | |
| نقطة الوميض | Non-flammable |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
6400 mg/kg (oral, rat)[3] |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Potassium ferricyanide |
كاتيونات أخرى
|
Sodium ferrocyanide Prussian blue |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
حديدوسيانيد الپوتاسيوم مركب كيميائي له الصيغة K4[Fe(CN)6]·3H2O ، ويكون على شكل بلورات صفراء .
في 20 فبراير 2002، تم اعتقال أربع مغاربة لحيازتهم خرائط مفصلة لسفارة الولايات المتحدة في روما ولشبكة إمداد روما بالمياه، وكذلك أربعة كيلوگرامات من فـِـرّوسيانيد البوتاسيوم.
التحضير
الإنتاج الحديث
Potassium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2[Fe(CN)6]·11H2O. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK2[Fe(CN)6], which in turn is treated with potassium carbonate to give the tetrapotassium salt.[4]
الإنتاج التاريخي
Historically, the compound was manufactured from organically derived nitrogenous carbon sources, iron filings, and potassium carbonate.[5] Common nitrogen and carbon sources were torrified horn, leather scrap, offal, or dried blood.
التفاعلات الكيميائية
Treatment of potassium ferrocyanide with nitric acid gives H2[Fe(NO)(CN)5]. After neutralization of this intermediate with sodium carbonate, red crystals of sodium nitroprusside can be selectively crystallized.[6]
Upon treatment with chlorine gas, potassium ferrocyanide converts to potassium ferricyanide:
- 2 K4[Fe(CN)6] + Cl2 → 2 K3[Fe(CN)6] + 2 KCl
This reaction can be used to remove potassium ferrocyanide from a solution.[بحاجة لمصدر]
A famous reaction involves treatment with ferric salts to give Prussian blue. With the approximate composition KFe2(CN)6, this insoluble but deeply coloured material is the blue of blueprinting.
التطبيقات
Potassium ferrocyanide finds many niche applications in industry. It and the related sodium salt are widely used as anticaking agents for both road salt and table salt. The potassium and sodium ferrocyanides are also used in the purification of tin and the separation of copper from molybdenum ores. Potassium ferrocyanide is used in the production of wine and citric acid.[4]
In the laboratory, potassium ferrocyanide is used to determine the concentration of potassium permanganate, a compound often used in titrations based on redox reactions. Potassium ferrocyanide is used in a mixture with potassium ferricyanide and phosphate buffered solution to provide a buffer for beta-galactosidase, which is used to cleave X-Gal, giving a bright blue visualization where an antibody (or other molecule), conjugated to Beta-gal, has bonded to its target.On reacting with Fe(3) it gives a Prussian blue colour. Thus it is used as an identifying reagent for iron in labs.
Potassium ferrocyanide can be used as a fertilizer for plants.[بحاجة لمصدر]
Prior to 1900 AD, before the invention of the Castner process, potassium ferrocyanide was the most important source of alkali metal cyanides.[4] In this historical process, potassium cyanide was produced by decomposing potassium ferrocyanide:[5]
K4[Fe(CN)6] → 4 KCN + FeC2 + N2
البنية
Like other metal cyanides, solid potassium ferrocyanide, both as the hydrate and anhydrous salts, has a complicated polymeric structure. The polymer consists of octahedral [Fe(CN)6]4− centers crosslinked with K+ ions that are bound to the CN ligands.[7] The K+---NC linkages break when the solid is dissolved in water.
السمية
Potassium ferrocyanide is nontoxic, and is not decomposed to cyanide in the body. The toxicity in rats is low, with lethal dose (LD50) at 6400 mg/kg.[2]
انظر أيضاً
المصادر
- ^ https://play.google.com/books/reader?printsec=frontcover&output=reader&id=gXwPAAAAYAAJ&pg=GBS.PA8
- ^ أ ب "POTASSIUM FERROCYANIDE MSDS Number: P5763 - Effective Date: 12/08/96". J. T. Baker Inc. Retrieved 2012-04-08.
- ^ http://chem.sis.nlm.nih.gov/chemidplus/rn/13943-58-3
- ^ أ ب ت Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. (October 2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub3.
- ^ أ ب Von Wagner, Rudolf (1897). Manual of chemical technology. New York: D. Appleton & Co. p. 474 & 477.
- ^ Seel, F. (1965). "Sodium Nitrosyl Cyanoferrate". In Brauer, G. (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 2 (2nd ed.). New York: Academic Press. p. 1768. LCCN 63-14307.
- ^ Willans, M.J.; Wasylishen, R.E.; McDonald, R. "Polymorphism of potassium ferrocyanide trihydrate as studied by solid-state multinuclear NMR spectroscopy and X-ray diffraction" Inorganic Chemistry 2009, volume 48, p4342-4353
وصلات خارجية
- "Cyanide (inorganic) compounds fact sheet". National Pollutant Inventory Australia.
