حديديسيانيد الپوتاسيوم
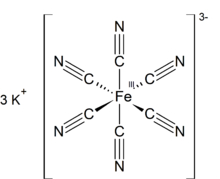
| |
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Potassium hexacyanoferrate(III)
| |
| أسماء أخرى
Red prussiate of Potash,
Prussian red, Potassium ferricyanide | |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.916 |
PubChem CID
|
|
| رقم RTECS |
|
CompTox Dashboard (EPA)
|
|
| الخصائص | |
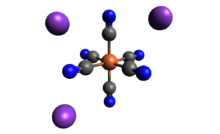
| الصيغة الجزيئية | K3[Fe(CN)6] |
| كتلة مولية | 329.24 g/mol |
| المظهر | deep red crystals, sometimes small pellets, orange to dark red powder |
| الكثافة | 1.89 g/cm3, solid |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 330 g/L ("cold water") 464 g/L (20 °C) 775 g/L ("hot water")[1] |
| قابلية الذوبان | slightly soluble in alcohol soluble in acid soluble in water |
| القابلية المغناطيسية | +2290.0·10−6 cm3/mol |
| البنية | |
| البنية البلورية | monoclinic |
| هندسة إحداثية |
octahedral at Fe |
| المخاطر | |
| صفحة بيانات السلامة | MSDS |
| توصيف المخاطر | R20, R21, R22, R32 |
| تحذيرات وقائية | S26, S36 |
| NFPA 704 (معيـَّن النار) | |
| نقطة الوميض | Non-flammable |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Potassium ferrocyanide |
كاتيونات أخرى
|
Prussian blue |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion.[2] It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin,[3] and was initially used in the production of ultramarine dyes.
Preparation
Potassium ferricyanide is manufactured by passing chlorine through a solution of potassium ferrocyanide. Potassium ferricyanide separates from the solution:
- 2 K4[Fe(CN)6] + Cl2 → 2 K3[Fe(CN)6] + 2 KCl
Structure
Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. The polymer consists of octahedral [Fe(CN)6]3− centers crosslinked with K+ ions that are bound to the CN ligands.[4] The K+---NCFe linkages break when the solid is dissolved in water.
Applications
The compound has widespread use in blueprint drawing and in photography (Cyanotype process). Several photographic print toning processes involve the use of potassium ferricyanide. Potassium ferricyanide was used as an oxidizing agent to remove silver from color negatives and positives during processing, a process called bleaching. Because potassium ferricyanide bleaches are environmentally unfriendly, short-lived and capable of releasing cyanide gas if mixed with acid, bleaches using ferric EDTA have been used in color processing since the 1972 introduction of the Kodak C-41 process. In color lithography, potassium ferricyanide is used to reduce the size of color dots without reducing their number, as a kind of manual color correction called dot etching. It is also used in black-and-white photography with sodium thiosulfate (hypo) to reduce the density of a negative or gelatin silver print where the mixture is known as Farmer's reducer; this can help offset problems from overexposure of the negative, or brighten the highlights in the print.[5]
The compound is also used to harden iron and steel, in electroplating, dyeing wool, as a laboratory reagent, and as a mild oxidizing agent in organic chemistry.
Potassium ferricyanide is also one of two compounds present in ferroxyl indicator solution (along with phenolphthalein) which turns blue (Prussian blue) in the presence of Fe2+ ions, and which can therefore be used to detect metal oxidation that will lead to rust. It is possible to calculate the number of moles of Fe2+ ions by using a colorimeter, because of the very intense color of Prussian blue Fe4[Fe(CN)6]3.
Potassium ferricyanide is often used in physiology experiments as a means of increasing a solution's redox potential (E°' ~ 436 mV at pH 7). As such, it can oxidize reduced cytochrome c (E°' ~ 247 mV at pH 7) in intact isolated mitochondria. Sodium dithionite is usually used as a reducing chemical in such experiments (E°' ~ −420 mV at pH 7).
Potassium ferricyanide is used in many amperometric biosensors as an electron transfer agent replacing an enzyme's natural electron transfer agent such as oxygen as with the enzyme glucose oxidase. It is used as this ingredient in many commercially available blood glucose meters for use by diabetics.
Potassium ferricyanide is combined with potassium hydroxide (or sodium hydroxide as a substitute) and water to formulate Murakami's etchant. This etchant is used by metallographers to provide contrast between binder and carbide phases in cemented carbides.
Prussian blue
Prussian blue, the deep blue pigment in blue printing, is generated by the reaction of K3[Fe(CN)6] with ferrous (Fe2+) ions as well as K4[Fe(CN)6] with ferric salts.[6]
In histology, potassium ferricyanide is used to detect ferrous iron in biological tissue. Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbull's blue or Prussian blue. To detect ferric (Fe3+) iron, potassium ferrocyanide is used instead in the Perls' Prussian blue staining method.[7] The material formed in the Turnbull's blue reaction and the compound formed in the Prussian blue reaction are the same.[8][9]
Safety
Potassium ferricyanide has low toxicity, its main hazard being that it is a mild irritant to the eyes and skin. However, under very strongly acidic conditions, highly toxic hydrogen cyanide gas is evolved, according to the equation:
- 6 H+ + [Fe(CN)6]3− → 6 HCN + Fe3+[10]
The reaction with hydrochloric acid is as follows:
- 6 HCl + K3[Fe(CN)6] → 6 HCN + FeCl3 + 3 KCl
See also
الهامش
- ^ Kwong, H.-L. (2004). "Potassium Ferricyanide". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289.
- ^ Sharpe, A. G. (1976). The Chemistry of Cyano Complexes of the Transition Metals. London: Academic Press.
- ^ Ihde, A.J. (1984). The Development of Modern Chemistry (2nd ed.). New York: Dover Publications. p. 153.
- ^ Figgis, B.N.; Gerloch, M.; Mason, R. "The crystallography and paramagnetic anisotropy of potassium ferricyanide" Proceedings of the Royal Society of London, Series A: Mathematical and Physical Sciences 1969, vol. 309, p91-118. doi:10.1098/rspa.1969.0031
- ^ Stroebel, L.; Zakia, R. D. (1993). "Farmer's Reducer". The Focal Encyclopedia of Photography. Focal Press. p. 297. ISBN 978-0-240-51417-8.
- ^ Dunbar, K. R.; Heintz, R. A. (1997). "Chemistry of Transition Metal Cyanide Compounds: Modern Perspectives". Progress in Inorganic Chemistry. Vol. 45. pp. 283–391. doi:10.1002/9780470166468.ch4.
- ^ Carson, F. L. (1997). Histotechnology: A Self-Instructional Text (2nd ed.). Chicago: American Society of Clinical Pathologists. pp. 209–211. ISBN 0-89189-411-X.
- ^ Tafesse, F. (2003). "Comparative Studies on Prussian Blue or Diaquatetraamine-Cobalt(III) Promoted Hydrolysis of 4-Nitrophenylphosphate in Microemulsions" (pdf). International Journal of Molecular Sciences. 4 (6): 362–370. doi:10.3390/i4060362.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Verdaguer, M.; Galvez, N.; Garde, R.; Desplanches, C. (2002). "Electrons at Work in Prussian Blue Analogues" (pdf). Electrochemical Society Interface. 11 (3): 28–32. doi:10.1002/chin.200304218.
- ^ "MSDS for potassium ferricyanide" (PDF).
وصلات خارجية
- CS1 maint: unflagged free DOI
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- مركبات الپوتاسيوم
- Iron compounds
- Cyano complexes
- Coordination compounds
- كيماويات التصوير الضوئي
- عوامل مؤكسدة
