نيتروسونيوم
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك النظامي
Oxidonitrogen(1+)[1] | |||
| أسماء أخرى
Nitrosonium
Iminooxidanium | |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| اختصارات | NO(+) | ||
| ChEBI | |||
| ChemSpider | |||
| مرجع Gmelin | 456 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
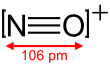
The nitrosonium ion is NO+
, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO
4, NOSO
4H (nitrosylsulfuric acid, more descriptively written ONSO
3OH) and NOBF
4. The ClO−
4 and BF−
4 salts are slightly soluble in acetonitrile CH
3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
NO+
is isoelectronic with CO, CN−
and N
2. It arises via protonation of nitrous acid:
- HONO + H+
 NO+ + H2O
NO+ + H2O
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Chemical properties
Hydrolysis
NO+
reacts readily with water to form nitrous acid:
- NO+ + H
2O → HONO + H+
For this reason, nitrosonium compounds must be protected from water or even moist air. With base, the reaction generates nitrite:
- NO+ + 2 NaOH → NaNO
2 + Na+ + H
2O
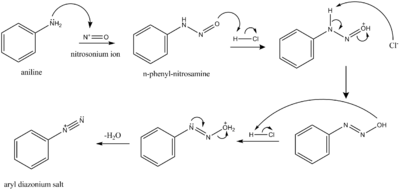
As a diazotizing agent
NO+
reacts with aryl amines, ArNH
2, to give diazonium salts, ArN+
2. The resulting diazonium group is easily displaced (unlike the amino group) by a variety of nucleophiles.
As an oxidizing agent
NO+
, e.g. as NOBF
4, is a strong oxidizing agent:[2]
- vs. ferrocene/ferrocenium, [NO]+
in CH
2Cl
2 solution has a redox potential of 1.00 V (or 1.46–1.48 V vs SCE), - vs. ferrocene/ferrocenium, [NO]+
in CH
3CN solution has a redox potential of 0.87 V vs. (or 1.27–1.25 V vs SCE).
NOBF
4 is a convenient oxidant because the byproduct NO is a gas, which can be swept from the reaction using a stream of N
2. Upon contact with air, NO forms NO
2, which can cause secondary reactions if it is not removed. NO
2 is readily detectable by its characteristic orange color.
Nitrosylation of arenes
Electron-rich arenes are nitrosylated using NOBF4.[3] One example involves anisole:
- CH3OC6H5 + NOBF4 → CH3OC6H4NO + HBF4
Nitrosonium, NO+
, is sometimes confused with nitronium, NO+2, the active agent in nitrations. These species are quite different, however. Nitronium is a more potent electrophile than is nitrosonium, as anticipated by the fact that the former is derived from a strong acid (nitric acid) and the latter from a weak acid (nitrous acid).
As a source of nitrosyl complexes
NOBF4 reacts with some metal carbonyl complexes to yield related metal nitrosyl complexes.[4] One must be careful that [NO]+ is transferred vs. electron transfer (see above).
- (C6Et6)Cr(CO)3 + NOBF4 → [(C6Et6)Cr(CO)2(NO)]BF4 + CO
انظر أيضاً
المصادر
- ^ Nomenclature of Inorganic Chemistry : IUPAC Recommendations 2005 (Red Book). Cambridge: The Royal Society of Chemistry. 2005. p. 315. ISBN 978-0-85404-438-2.
- ^ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ^ E. Bosch and J. K. Kochi. "Direct Nitrosation of Aromatic Hydrocarbons and Ethers with the Electrophilic Nitrosonium Cation". Journal of Organic Chemistry, 1994, volume 59, pp. 5573–5586.
- ^ T. W. Hayton, P. Legzdins, W. B. Sharp. "Coordination and Organometallic Chemistry of Metal-NO Complexes". Chemical Reviews 2002, volume 102, pp. 935–991.