فلوريد المنغنيز الثلاثي
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Manganese(III) fluoride
| |
| أسماء أخرى
Manganese trifluoride, manganic fluoride
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.096 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | MnF3 |
| كتلة مولية | 111.938 g/mol |
| المظهر | purple-pink powder hygroscopic |
| الكثافة | 3.54 g/cm3 |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | hydrolysis |
| القابلية المغناطيسية | +10,500·10−6 cm3/mol |
| البنية | |
| البنية البلورية | Monoclinic, mS48 |
| الزمرة الفراغية | C2/c, No. 15 |
| هندسة إحداثية |
distorted octahedral |
| المخاطر | |
| خطر رئيسي | toxic fumes |
| ن.م.ع. مخطط تصويري |  
|
| ن.م.ع. كلمة الاشارة | danger |
| H272, H301, H312, H315, H319, H332, H335 | |
| P220, P261, P280, P301+P310, P305+P351+P338 | |
| مركبات ذا علاقة | |
أنيونات أخرى
|
manganese(III) oxide, manganese(III) acetate |
كاتيونات أخرى
|
chromium(III) fluoride, iron(III) fluoride. cobalt(III) fluoride |
مركـّبات ذات علاقة
|
manganese(II) fluoride, manganese(IV) fluoride |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
فلوريد المنغنيز الثلاثي Manganese(III) fluoride (ويـُعرف أيضاً بإسم ثالث فلوريد المنغنيز Manganese trifluoride ) هو مركب غير عضوي صيغته MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent.[2] It forms a hydrate and many derivatives.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التخليق والبنية والتفاعلات
MnF3 can be prepared by treating a solution of MnF2 in hydrogen fluoride with fluorine:[3]
- MnF2 + 0.5 F2 → MnF3
It can also be prepared by the reaction of elemental fluorine with a manganese(II) halide at ~250 °C.[4]
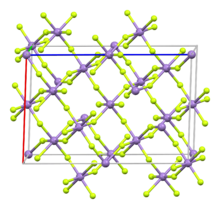
البنية
Like vanadium(III) fluoride, MnF3 features octahedral metal centers with the same average M-F bond distances. In the Mn compound, however, is distorted (and hence a monoclinic unit cell vs. a higher symmetry one) due to the Jahn-Teller effect, with pairs of Mn-F distances of 1.79, 1.91, 2.09 Å.[5][6][7]
The hydrate MnF3.3H2O is obtained by crystallisation of MnF3 from hydrofluoric acid. The hydrate exists as two polymorphs, with space groups P21/c and P21/a. Each consists of the salt [Mn(H2O)4F2]+[Mn(H2O)2F4]− ).[8]
Reactions
MnF3 is Lewis acidic and forms a variety of derivatives. One example is K2MnF3(SO4).[9] MnF3 reacts with sodium fluoride to give the octahedral hexafluoride:[4]
- 3NaF + MnF3 → Na3MnF6
Related reactions salts of the anions MnF52− or MnF4−. These anions adopt chain and layer structures respectively, with bridging fluoride. Manganese remains 6 coordinate, octahedral, and trivalent in all of these materials.[4]
Manganese(III) fluoride fluorinates organic compounds including aromatic hydrocarbons,[10] cyclobutenes,[11] and fullerenes.[12]
On heating, MnF3 decomposes to manganese(II) fluoride.[13][14]
MnF3 is a source of MnCl3 complexes by reaction with bismuth trichloride.[15]
انظر أيضاً
- CoF3, another fluorinating agent based on a transition metal in an oxidising +3 state.
المراجع
- ^ GHS: sigma-aldrich 339296[dead link]
- ^ Burley, G. A.; Taylor, R. (2004). "Manganese(III) Fluoride". Encyclopedia of Reagents for Organic Synthesis. J. Wiley & Sons. doi:10.1002/047084289X.rn00411.
- ^ Z. Mazej (2002). "Room temperature syntheses of MnF3, MnF4 and hexafluoromanganete(IV) salts of alkali cations". Journal of Fluorine Chemistry. 114 (1): 75–80. doi:10.1016/S0022-1139(01)00566-8.
- ^ أ ب ت Inorganic chemistry, Catherine E. Housecroft, A.G. Sharpe, pp.711-712, section Manganese (III) , googlebooks link
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Hepworth, M. A.; Jack, K. H.; Nyholm, R. S. (1957). "Interatomic Bonding in Manganese Trifluoride". Nature. 179 (4552): 211–212. Bibcode:1957Natur.179..211H. doi:10.1038/179211b0. S2CID 4208409.
- ^ M. A. Hepworth; K. H. Jack (1957). "The Crystal Structure of Manganese Trifluoride, MnF3". Acta Crystallographica. 10 (5): 345–351. doi:10.1107/S0365110X57001024.
- ^ Molinier Michel; Massa Werner (1992). "Structures of two polymorphs of MnF3·3H2O". Journal of Fluorine Chemistry. 57 (1–3): 139–146. doi:10.1016/S0022-1139(00)82825-0.
- ^ Bhattacharjee, M. N; Chaudhuri, M. K. (1990). Dipotassium Trifluorosulfatomanganate(III). Inorganic Syntheses. Vol. 27. pp. 312–313. doi:10.1002/9780470132586.ch61. ISBN 9780470132586.
{{cite book}}:|journal=ignored (help) - ^ Fluorination of p-chlorobenzotrifluoride by manganese trifluoride Archived 2011-08-23 at the Wayback Machine A. Kachanov, V. Kornilov, V.Belogay , Fluorine Notes :Vol. 1 (1) November–December 1998 , via notes.fluorine1.ru
- ^ "Fluorination of Fluoro-Cyclobutene with High-Valency Metal Fluoride". Journal of Fluorine Chemistry. 127: 79–84. 2006. doi:10.1016/j.jfluchem.2005.10.007.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Fluorination of the Cubic and Hexagonal C60 Modifications by Crystalline Manganese Trifluoride". Physics of the Solid State. 44 (4): 629–630. 2002. Bibcode:2002PhSS...44..629A. doi:10.1134/1.1470543. S2CID 94250136.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^
 Chisholm, Hugh, ed. (1911). . دائرة المعارف البريطانية. Vol. 17 (eleventh ed.). Cambridge University Press. p. 570.
Chisholm, Hugh, ed. (1911). . دائرة المعارف البريطانية. Vol. 17 (eleventh ed.). Cambridge University Press. p. 570. {{cite encyclopedia}}: Cite has empty unknown parameter:|coauthors=(help) - ^ In situ time-resolved X-ray diffraction study of manganese trifluoride thermal decomposition , J.V. Raua, V. Rossi Albertinib, N.S. Chilingarova, S. Colonnab, U. Anselmi Tamburini, Journal of Fluorine Chemistry 4506 (2001) 1–4 , online version
- ^ Nachtigall, Olaf; Pataki, Astrid; Molski, Matthias; Lentz, Dieter; Spandl, Johann (2015). "Solvates of Manganese Trichloride Revisited - Synthesis, Isolation, and Crystal Structure of MnCl3(THF)3". Zeitschrift für Anorganische und Allgemeine Chemie. 641 (6): 1164–1168. doi:10.1002/zaac.201500106.
للاستزادة
- Novel syntheses of some binary fluorides: the role of anhydrous hydrogen fluoride Acta Chim. Slov. 1999, 46(2), pp. 229–238, Zoran Mazej, Karel Lutar and Boris Žemva
- Knudsen Cell mass spectrometry study of Manganese Trifluoride vaporisation, High temperature corrosion and materials chemistry IV: proceedings of the International Symposium, pp. 521–525, google books
وصلات خارجية
- Articles with dead external links from December 2021
- CS1 errors: periodical ignored
- CS1 errors: deprecated parameters
- مقالات المعرفة المحتوية على معلومات من دائرة المعارف البريطانية طبعة 1911
- Short description matches Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- فلوريدات
- مركبات المنغنيز الثلاثية
- هاليدات فلزية
- عوامل فلورة