فولرين
| جزء من سلسلة مقالات عن |
| المواد النانوية |
|---|
 |
| الأنابيب النانوية الكربونية |
| الفولرينات |
| جسيمات النانو الأخرى |
| المواد نانوية البنية |
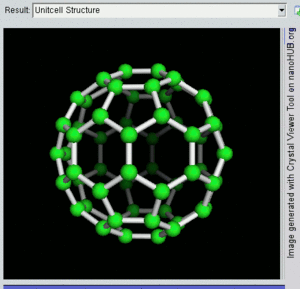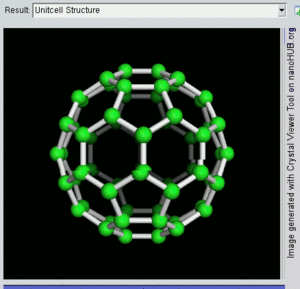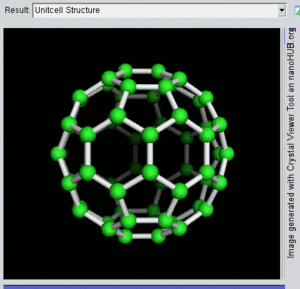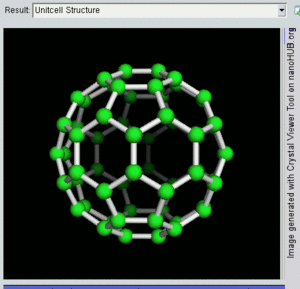
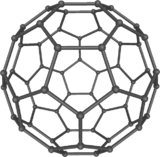
فولرين Fullerene هو أي جزيء مكون بالكامل من الكربون، على شكل كرة مجوفة أو سطح ناقص أو أنبوب. الفولرينات الكروية تسمى أيضا كرات بكي buckyballs، أما الأسطوانية فتدعى أنابيب النانو الكربونية أو أنابيب بكي. الجرافين هو مثل صفحة فولرينية مسطحة. ويشبه الفولرين في تركيبه الگرافيت، الذى يتكون من صحائف مكدسة من الگرافين لحلقات سداسية الأضلاع مترابطة؛ إلا أنها قد تتضمن أيضا حلقات خماسية الأضلاع (وأحياناً ثمانية الأضلاع). تلك الحلقات من شأنها الحيلولة دون تحول الصفحات الى شكل مسطح.
أول فولرين تم اكتشافه، وأعطى اسمه للعائلة كلها، كان بكمنسترفولرين (C60) الذي حضـَّره في عام 1985 ريتشارد سمالي وروبرت كرل وجيمس هيث وشون اوبراين وهارولد كروتو في جامعة رايس. الاسم كان لتكريم ريتشارد بكمنستر فولر، المصمم المعماري الشهير، الذي صمم القبة الجيوديسية التي تشبه الفولرين. ومنذ ذلك الحين فقد اُكتـُشِف أن الفولرين يتواجد (ولو بندرة) في الطبيعة.[1]
زاد اكتشاف الفولرين بقدر هائل عدد الأشكال المتآصلة المعروفة من الكربون، والتي كانت حتى ذلك الحين مقصورة على الگرافيت والألماس والكربون اللا بلوري مثل السناج (الهباب) والفحم النباتي. وقد كانت كرات وأنابيب بكي موضع بحث مكثف، وذلك لما يتميزون به من كيمياء فريدة ولتطبيقاتهم التكنولوجية، وخصوصاً في علم المواد والإلكترونيات والتقانة النانوية.
التنبؤ والاكتشاف

القفص متساوي الأوجه C60H60 كان قد ذُكِر في 1965 كبنية طبولوجية ممكنة.[2] وجود C60 كان قد تنبأ به إيـْجي اوساوا من جامعة تويوهاشي للتكنولوجيا في 1970.[3][4] وقد لاحظ أن بنية جزيء كورانيولين corannulene كانت جزءا من شكل كرة قدم، كما افترض أن كشل كرة قدم كاملة يمكن أيضاً أن يتواجد. وقد ظهرت فكرته في المجلات اليابانية، إلا أنها لم تصل اوروبا أو الأمريكتين.
وأيضاً في 1970، اقترح ر. و. هنسون (وكان وقتها في هيشة أبحاث الطاقة الذرية) البنية وطور نموذجاً لـ C60. الدليل على هذه الصيغة الجديدة من الكربون كان ضعيفاً جداً ولم يـُقبل، حتى من زملائه. والنتائج لم تـُنشر قط، ولكن أشير إلها في دورية كربون في 1999.[5][6]
مع الإستعانة بكتلة الطيف، قمم منفصلة قد شوهدت المقابلة للجزيئات مع الكتلة الفعلية لستون أو سبعون أو أكثر من ذرات الكربون. في 1985, هارولد كروتو (حينئذ في جامعة سسكس)، جيمس .ر هيث , شين اوبرين، روبرت كرل و ريتشارد سمالي , من جامعة رايس، إكتشفوا C60، وفى نهاية الأمر توصلوا إلى إكتشاف الفولرينات.[7] كروتو, كرل وسمالى قد منحوا جائزة جائزة نوبل في الكيمياء لدورهم في اكتشاف هذا النوع من المركبات. C60 والفولرينات الأخرى في وقت لاحق لوحظ وجودها خارج المختبر (فعلى سبيل المثال، الشمعة العادية و هباب). حتى 1991, كان من السهولة الحصول على عينات تقاس بالجرام من مسحوق الفولرين بإستخدام تقنية دونالد هوفمان وڤولفگانگ كراتشمير. تنقية الفوليرينات بقيت تمثل تحديا للعلماء والباحثين , وكانت تشكل وتؤثر في أسعار تلك المادة . وقد سميت داخل شبكة الفولرين تمتلك الأيونات أو الجزيئات الصغيرة وقد أدرجت داخل قفص ذرات الفولرينات. وهى غير عادية في العديد من التفاعلات التفاعلات العضوية مثل تفاعل بينجيل المكتشف عام 1993. وكانت أولى الأنابيب النانوية قد تم الحصول عليها في 1991.[8] شذرات قليلة من الفولرين, على هيئة C60, C70, C76, و C84 جزيئات, توجد في الطبيعة، مختبئة داخل السخام وتتكون من نواتج الإشتعال في الغلاف الجوى.[9] Recently, بكمنستر فولرين تتواجد في عائلة من المعادن, تعرف بإسم شينجات في كارليا, روسيا.[بحاجة لمصدر] تواجدC60 تنبأ به إياجى أوساوا تابع جامعة تويوهاشى للتكنولوجيا في مجلة يابانية [10] في 1970. هو لاحظ أن البنيةالخاصة بجزىء كورابيولين كان مشابها لكرة -قدم شكل, وهو قد إفترض أن شكل الكرة الكامل, يمكن أيضا وجوده . وأرسلت تلك الفكرة الى مجلة يابانية, و لكنها لم تصل الى أوروبا أو أميريكا.
التسمية
بكمنسترفولرين (C60) تم تسميتها نسبة الى ريتشارد بكمنستر فولر, مصمم نماذج معمارية بارز الذى جعل القبة الجيوديسية أكثر شعبية. وحيث أن بكمنستر فولرين لها شكل مشابه لهذا الشكل من القباب, فالإسم بدا أكثر من مناسب. و حيث أن إكتشاف عائلة الفولرين جاء بعد بكمنستر فولرين, فإن الإسم المختصر 'فولرين' أستخدم للإشارة الى عائلة الفولرينات.
للرسومات الخاصة القبة الجيوديسية انظر مونتريال المحيط الحيوى و مشروع عدن , حديقة ميسوري للنباتات , عالم العلوم TELUS في العلوم العالمية, بارك ميتشل للمحمية النباتية , القبة الذهبية, قبة تاكوما, برج ريونيون, و مركبة الفضاء الأرض (Epcot). ref>http://machinedesign.com/ContentItem/60618/Anewbuckyballbouncesintotown.aspx</ref>
كميات ضئيلة, من C60, C70, C76, و C84 جزيئات تنتج في الطبيعة متخفية في السخامو تتكون من نواتج الإحتراق في الغلاف الجوى .[11] وفى الوقت الحالى, فوليرينات باكمنستر وجدت في عائلة من المعادن يطلق عليها الشينجايتات في كارليا، روسيا.
إن وجود C60</> كان قد تنبأ به إيجي أوساوا تابع لجامعة تويوهاشى للتكنولوجيا في مجلة يابانية[12] . و هو قد لاحظ أن تركيب جزيى كورانيولين كان مرادفا لشكل كرة القدم, ووضع فرضية تفيد أن الشكل الكامل للكرة يمكن أيضا أن يوجد. و نشر هذا التقرير في مجلة يابانية, و لكن لم يصل الى أوروبا أو أميريكا.
تنويعات
منذ إكتشاف الفولرينات في 1985, تنويعات في التركيب برزت الى الوجود, خارج نطاق العناقيد الفردية نفسها. والأمثلة تشمل:[13]
- عناقيد كرات باكي : أصغر عضو C 20 (الصورة غير المشبعة منإثنى عشرى الأوجه) و الأكثر شيوعاC 60;
- الأنابيب النانوية: أنابيب مجوفة بأبعاد صغيرة جداً، ذات جدار واحد أو متعدد؛ تطبيقات محتملة في صناعة الإلكترونيات؛
- أنابيب كبيرة megatubes: أكبر قطراً من الأنابيب النانوية وprepared with walls of different thickness; potentially used for the transport of a variety of molecules of different sizes;[14]
- پوليمرات: سلسلة، بوليمرات ثنائية وثلاثية الأبعاد تتكون تحت ظروف ضغط عالي ودرجة حرارة عالية
- "بصل" نانوي: spherical particles based on multiple carbon layers surrounding a buckyball core; proposed for lubricants;[15]
- عـُملات "كرة وسلسلة" مرتبطة: كـُرَتا بكي مرتبطتان بسلسلة كربونية؛[16]
- حلقات فولرين[17]
"كرات بكي"

بكمنستر فولرين (IUPAC إسم (C60-Ih)[5,6]fullerene) هو أصغر جزيىء فولرين حيث إثنان من خماسى الأوجه تشترك في حافة واحدة (التي يمكن أن تزعزع الاستقرار , كما في بنتالين). وهو أيضا أكثر شيوعافى الطبيعة, حيث يوجد غالبا في السخام.
إن تركيب C60 هو مبتور (T = 3) العشروني الوجوه, و هو يشبه كرة القدم من نوع من اثني عشر والعشرين خماسي وسداسي الأوجه, حيث ذرة الكربون في كل القمم المضلعة وإلى جانب كل الروابط في كل حافة.
The فان دير ڤال قطرها للكربون C60 الجزىء 1 نانومتر (نم). النواة الى قطر النواة لجزىء للكربون C60 هو حوالى õ 0.7 nm.
ذرة C60 الجزىء له رابطين طول. 6:6 رابط حلقى (بين إثنان من سداسى الأوجه) يمكن إعتباره "روابط زوجية" و هى أقصر 6:5 روابط (بين سداسى الأوجه وخماسى الأوجه ). و متوسط طول رابطته هو 1.4 أنجستروم.
كرات بكي بورونية
A new type of buckyball utilizing boron atoms instead of the usual carbon has been predicted and described by researchers at Rice University. The B-80 structure is predicted to be more stable than the C-60 buckyball.[18] One reason for this given by the researchers is that the B-80 is actually more like the original geodesic dome structure popularized by Buckminster Fuller which utilizes triangles rather than hexagons. However, this work has been subject to much criticism by quantum chemists [19][20] as it was concluded that the predicted Ih symmetric structure was vibrationally unstable and the resulting cage undergoes a spontaneous symmetry breaking yielding a puckered cage with rare Th symmetry (symmetry of the volleyball) [21] number of six rings of carbon in this molecule is 20 and number of five member rings is 12
تنويعات من كرات بكي
وهناك بكمنسترفولرين شائع آخر هو C70,[22] إلا أن الفولرينات ذوات 72, 76, 84 وحتى 100 ذرة كربون يشيع الحصول عليهم.
وفي المصطلحات الرياضياتية، فبنية الفولرين هي trivalent convex polyhedron بأوجه خماسية الأضلاع وسداسية. In graph theory, the term fullerene refers to any 3-regular, planar graph with all faces of size 5 or 6 (including the external face). It follows from Euler's polyhedron formula, |V|-|E|+|F| = 2, (where |V|, |E|, |F| indicate the number of vertices, edges, and faces), that there are exactly 12 pentagons in a fullerene and |V|/2-10 hexagons.
|
|

|
|
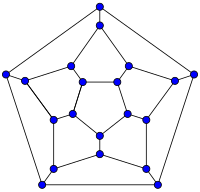
| 20-fullerene (dodecahedral graph) |
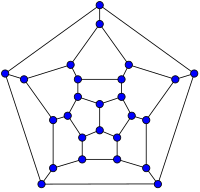
26-fullerene graph | 60-fullerene (truncated icosahedral graph) |
70-fullerene graph |
أصغر فولرين هو dodecahedron--the unique C20. There are no fullerenes with 22 vertices.[23] The number of fullerenes C2n grows with increasing n = 12,13,14..., roughly in proportion to n9 (المتتالية A007894 في OEIS). For instance, there are 1812 non-isomorphic fullerenes C60. Note that only one form of C60, the buckminsterfullerene alias truncated icosahedron, has no pair of adjacent pentagons (أصغر تلك الأنواع من الفولرينات). To further illustrate the growth, there are 214,127,713 non-isomorphic fullerenes C200, 15,655,672 of which have no adjacent pentagons.
المواد النانوية الكربونية على شكل Trimetasphere اكتشفهم الباحثون في ڤرجينيا تك and licensed exclusively to Luna Innovations. This class of novel molecules comprises 80 carbon atoms (C80) forming a sphere which encloses a complex of three metal atoms and one nitrogen atom. These fullerenes encapsulate metals which puts them in the subset referred to as metallofullerenes. Trimetaspheres have the potential for use in diagnostics (as safe imaging agents), therapeutics and in organic solar cells.
أنابيب الكربون النانوية
 مقالة مفصلة: أنبوب كربون نانوي
مقالة مفصلة: أنبوب كربون نانوي
Nanotubes are cylindrical fullerenes. These tubes of carbon are usually only a few nanometres wide, but they can range from less than a micrometer to several millimeters in length. They often have closed ends, but can be open-ended as well. There are also cases in which the tube reduces in diameter before closing off. Their unique molecular structure results in extraordinary macroscopic properties, including high tensile strength, high electrical conductivity, high ductility, high resistance to heat, and relative chemical inactivity (as it is cylindrical and "planar" — that is, it has no "exposed" atoms that can be easily displaced). One proposed use of carbon nanotubes is in paper batteries, developed in 2007 by researchers at Rensselaer Polytechnic Institute.[24] Another proposed use in the field of space technologies and science fiction is to produce high-tensile carbon cables required by a space elevator.
Carbon nanobuds
Nanobuds have been obtained by adding Buckminsterfullerenes to carbon nanotubes.
الخصائص
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. Popular Science has published articles about the possible uses of fullerenes in armor.[بحاجة لمصدر] In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated antimicrobial agents.[25]
In the field of nanotechnology, heat resistance and superconductivity are some of the more heavily studied properties.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By DFT and TD-DFT methods one can obtain IR, Raman and UV spectra. Results of such calculations can be compared with experimental results.
العطرية
Researchers have been able to increase the reactivity of fullerenes by attaching active groups to their surfaces. The Buckminsterfullerene does not exhibit "superaromaticity:" that is, the electrons in the hexagonal rings do not delocalize over the whole molecule.
A spherical fullerene of n carbon atoms has n pi-bonding electrons. These should try to delocalize over the whole molecule. The quantum mechanics of such an arrangement should be like one shell only of the well-known quantum mechanical structure of a single atom, with a stable filled shell for n = 2, 8, 18, 32, 50, 72, 98, 128, etc.; i.e. twice a perfect square; but this series does not include 60. As a result, C60 in water tends to pick up two more electrons and become an anion. The nC60 described below may be the result of C60 trying to form a loose metallic bonding.
الكيمياء
 مقالة مفصلة: كيمياء الفولرين
مقالة مفصلة: كيمياء الفولرين
Fullerenes are stable, but not totally unreactive. The sp2-hybridized carbon atoms, which are at their energy minimum in planar graphite, must be bent to form the closed sphere or tube, which produces angle strain. The characteristic reaction of fullerenes is electrophilic addition at 6,6-double bonds, which reduces angle strain by changing sp2-hybridized carbons into sp3-hybridized ones.[26] The change in hybridized orbitals causes the bond angles to decrease from about 120 degrees in the sp2 orbitals to about 109.5 degrees in the sp3 orbitals. This decrease in bond angles allows for the bonds to bend less when closing the sphere or tube, and thus, the molecule becomes more stable.
Other atoms can be trapped inside fullerenes to form inclusion compounds known as endohedral fullerenes. An unusual example is the egg shaped fullerene Tb3N@C84, which violates the isolated pentagon rule.[27] Recent evidence for a meteor impact at the end of the Permian period was found by analyzing noble gases so preserved.[28] Metallofullerene-based inoculates using the rhonditic steel process are beginning production as one of the first commercially-viable uses of buckyballs.
قابلية الذوبان
Fullerenes are sparingly soluble in many solvents. Common solvents for the fullerenes include aromatics, such as toluene, and others like ثاني كبريتيد الكربون. Solutions of pure Buckminsterfullerene have a deep purple color. Solutions of C70 are a reddish brown. The higher fullerenes C76 to C84 have a variety of colors. C76 has two optical forms, while other higher fullerenes have several structural isomers. Fullerenes are the only known allotrope of carbon that can be dissolved in common solvents at room temperature.
Some fullerene structures are not soluble because they have a small band gap between the ground and excited states. These include the small fullerenes C28,[29] C36 and C50. The C72 structure is also in this class, but the endohedral version with a trapped lanthanide-group atom is soluble due to the interaction of the metal atom and the electronic states of the fullerene. Researchers had originally been puzzled by C72 being absent in fullerene plasma-generated soot extract, but found in endohedral samples. Small band gap fullerenes are highly reactive and bind to other fullerenes or to soot particles.
Solvents that are able to dissolve buckminsterfullerene (C60) are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.[30]
- 1-chloronaphthalene (51 mg/mL)
- 1-methylnaphthalene (33 mg/mL)
- 1,2-dichlorobenzene (24 mg/mL)
- 1,2,4-trimethylbenzene (18 mg/mL)
- tetrahydronaphthalene (16 mg/mL)
- carbon disulfide (8 mg/mL)
- 1,2,3-tribromopropane (8 mg/mL)
- bromoform (5 mg/mL)
- toluene (3 mg/ml)
- benzene (1.5 mg/ml)
- cyclohexane (1.2 mg/ml)
- carbon tetrachloride (0.4 mg/ml)
- chloroform (0.25 mg/ml)
- n-hexane (0.046 mg/ml)
- tetrahydrofuran (0.006 mg/ml)
- acetonitrile (0.004 mg/ml)
- methanol (0.00004 mg/ml)
- water (1.3x10-11 mg/mL)
Solubility of C60 in some solvents shows unusual behaviour due to existence of solvate phases (analogues of crystallohydrates). For example, solubility of C60 in benzene solution shows maximum at about 313 K. Crystallization from benzene solution at temperatures below maximum results in formation of triclinic solid solvate with four benzene molecules C60•4C6H6 which is rather unstable in air. Out of solution, this structure decomposes into usual fcc C60 in few minutes' time. At temperatures above solubility maximum the solvate is not stable even when immersed in saturated solution and melts with formation of fcc C60. Crystallization at temperatures above the solubility maximum results in formation of pure fcc C60. Large millimetre size crystals of C60 and C70 can be grown from solution both for solvates and for pure fullerenes.[31][32]
ميكانيكا الكم
In 1999, researchers from the University of Vienna demonstrated that wave-particle duality applied to molecules such as fullerene.[33] One of the co-authors of this research, Julian Voss-Andreae, has since created several sculptures symbolizing wave-particle duality in fullerenes (see Fullerenes in popular culture for more detail).
Science writer Marcus Chown stated on the CBC radio show Quirks and Quarks in May 2006 that scientists are trying to make buckyballs exhibit the quantum behavior of existing in two places at once (quantum superposition).[34]
الأمان والسمية
When considering toxicological data, care must be taken to distinguish as necessary between what are normally referred to as fullerenes: (C60, C70,...); fullerene derivatives: C60 or other fullerenes with covalently bonded chemical groups; fullerene complexes (e.g., water-solubilized with surfactants, such as C60-PVP; host-guest complexes, such as with cyclodextrin; ), where the fullerene is physically bound to another molecule; C60 nanoparticles, which are extended solid-phase aggregates of C60 crystallites; and nanotubes, which are generally much larger (in terms of molecular weight and size) compounds, and are different in shape to the spheroidal fullerenes C60 and C70, as well as having different chemical and physical properties.
The above different compounds span the range from insoluble materials in either hydrophilic or lipophilic media, to hydrophilic, lipophilic, or even amphiphilic compounds, and with other varying physical and chemical properties. Therefore any broad generalization extrapolating for example results from C60 to nanotubes or vice versa is not possible, though technically all are fullerenes, as the term is defined as a close-caged all-carbon molecule. Any extrapolation of results from one compound to other compounds must take into account considerations based on a Quantitative Structural Analysis Relationship Study (QSARS), which mostly depends on how close the compounds under consideration are in physical and chemical properties.
In 1996[35] and 1997,[36] Moussa et al. studied the in vivo toxicity of C60 after intra-peritoneal administration of large doses. No evidence of toxicity was found and the mice tolerated a dose of 5 000 mg/kg of body weight (BW). Mori et al. (2006) [37] could not find toxicity in rodents for C60 and C70 mixtures after oral administration of a dose of 2 000 mg/kg BW and did not observe evidence of genotoxic or mutagenic potential in vitro. Other studies could not establish the toxicity of fullerenes: on the contrary, the work of Gharbi et al. (2005)[38] suggested that aqueous C60 suspensions failing to produce acute or subacute toxicity in rodents could also protect their livers in a dose-dependent manner against free-radical damage.
A comprehensive and recent review on fullerene toxicity is given by Kolosnjaj et al. (2007a,b, c).[39] [40] These authors review the works on fullerene toxicity beginning in the early 1990s to present, and conclude that very little evidence gathered since the discovery of fullerenes indicate that C60 is toxic.
With reference to nanotubes, a recent study of Poland et al. (2008)[41] on carbon nanotubes introduced into the abdominal cavity of mice led the authors to suggest comparisons to "asbestos-like pathogenicity". It should be noted that this was not an inhalation study, though there have been several performed in the past, therefore it is premature to conclude that nanotubes should be considered to have a toxicological profile similar to asbestos. Conversely, and perhaps illustrative of how the various classes of compounds which fall under the general term fullerene cover a wide range of properties, Sayes, et al., found that in vivo inhalation of C60(OH)24 and nano-C60 in rats gave no effect, whereas in comparison quartz particles produced an inflammatory response under the same conditions (Nano Letters, 2007, Vol. 7, No. 8, 2399-2406). As stated above, nanotubes are quite different in chemical and physical properties to C60, i.e., molecular weight, shape, size, physical properties (such as solubility) all are very different, so from a toxicological standpoint, different results for C60 and nanotubes are not suggestive of any discrepancy in the findings.
الموصلية الفائقة
After the synthesis of macroscopic amounts of fullerenes,[42] their physical properties could be investigated. Very soon Haddon et al.,[43] found that intercalation of alkali-metal atoms in solid C60 leads to metallic behavior.[44] In 1991, It was revealed that potassium-doped fullerenes becomes superconducting at 18K.[45] This was the highest transition temperature for a molecular superconductor. Since then, superconductivity has been reported in fullerene doped with various metals as well as potassium.[46][47][48] It is shown that the superconducting transition temperature in alkaline-metal-doped fullerene increase in the unit cell volume V.[49][50] As cesium forms the largest alkali ion, cesium doped fullerene is an important material in this family. Recently, superconductivity at 38K is reported in bulk Cs3C60 [51], but only under applied pressure. The highest superconducting transition temperature of 33 K at ambient pressure is reported for Cs2RbC60 [52].
The increase of transition temperature with the unit-cell volume had been believed to be an evidence for the BCS mechanism of C60 solid superconductivity, because inter C60 separation can be related to an increase in the density of states on the Fermi level, N(εF). Therefore, there have been many efforts to increasing the interfullerene separation. In particular, intercalating neutral molecules into the A3 C60 lattice to increase the interfullerene spacing while the valence of C60 is kept unchanged. However, this ammoniation technique has revealed a new aspect of fullerene intercalation compound: the Mott-Hubbard transition and the correlation between the orientation/orbital order of C60 molecules and the magnetic structure.[53]
The C60 molecules compose a solid of weakly bound molecules. The fullerites are therefore molecular solids, in which the molecular properties still survive. The discrete levels of a free C60 molecule are only weakly broadened in the solid, which leads to a set of essentially nonoverlapping bands with a narrow width of about 0.5 eV.[54] For an undoped C60 solid, the 5-fold hu band is the HOMO level, and the 3-fold t1u band is the empty LUMO level, and this system is a band insulator. But when the C60 solid is doped with metal atoms, the metal atoms give electrons to the t1u band or the upper 3-fold t1g band.[55] This partial electron occupation of the band leads to sometimes metallic behavior. However, A4C60 is an insulator, although the t1u band is only partially filled and should be a metal according to band theory.[56][57] this unpredicted behavior may be explained by the Jahn-Teller effect, where spontaneous deformations of high-symmetry molecules induce the splitting of degenerate levels to gain the electronic energy. The Jahn-Teller type electron-phonon interaction is strong enough in C60 solids to destroy the band picture for particular valence states.[58]
A narrow band or strongly correlated electronic system and degenerated ground states are important points to understand superconductivity in fullerene solids. When the inter-electron repulsion U is greater than the bandwidth, insulating localized electron ground state is produced in the simple Mott-Hubbard model. This explains the absence of superconductivity at ambient pressure in cesium-doped C60 solids.[59] Electron-correlation-driven localization of the t1u electrons exceeds the critical value, leading to the Mott-insulator. The application of high pressure decreases the interfullerene spacing, therefore cesium-doped C60 solids turns to metallic and superconducting.
A fully developed theory of C60 solids superconductivity is still lacking, but it has been widely accepted that strong electronic correlations and the Jahn-Teller electron-phonon coupling[60] produce local electron-pairings that show a high transition temperature close to the insulator-metal transition.[61]
التماكب الضوئي
Few fullerenes (e.g. C76, C78, C80, and C84) are inherently chiral because D2-symmetric and have been successfully resolved. Research efforts are ongoing to develop specific sensors for their enantiomers.
أمثلة للفولرينات في الثقافة الشعبية
الأمثلة للفولرينات في الثقافة الشعبية عديدة. Fullerenes appeared in fiction well before scientists took serious interest in them. In New Scientist there used to be a weekly column called "Daedalus" written by David Jones, which contained humorous descriptions of unlikely technologies. In 1966 the columnist included a description of C60 and other forms of graphite. This was meant as pure entertainment. Fullerenes are part of the plot in a science fiction novel named Decipher written by Stel Pavlou and published in 2001. Bucky Balls were also mentioned in A&E's 2008 remake of The Andromeda Strain, which is based on the Michael Crichton novel of the same name; John Ringo uses fullerene as a carrier for antimatter in his legacy of the aldenata series other novels to make mention of them include Kim Stanley Robinson's Mars Trilogy; Iron by Poul Anderson and Neal Stephenson's The Diamond Age. Buckyballs are referenced in similarity with a large spherical meteorite with the potential to unleash a virus that could kill all organic life on Earth In Clive Cussler's novel The Sacred Stone. Buckyball is the state molecule of Texas.[62]
فولريت (حالة صلبة)

Fullerites are the solid-state manifestation of fullerenes and related compounds and materials.
Polymerized single-walled nanotubes (P-SWNT) are a class of fullerites and are comparable to diamond in terms of hardness. However, due to the way that nanotubes intertwine, P-SWNTs do not have the corresponding crystal lattice that makes it possible to cut diamonds neatly. This same structure results in a less brittle material, as any impact that the structure sustains is spread out throughout the material. Because nanotubes are still very expensive to produce in useful quantities, uses for a material lighter and stronger than steel will have to wait until nanotube production becomes more economically viable.[بحاجة لمصدر]
فولريت فائق الصلابة، كرة بكي
"Ultrahard fullerite" is a coined term frequently used to describe material produced by high-pressure high-temperature (HPHT) processing of fullerite. Such treatment converts fullerite into a nanocrystalline form of diamond which exhibits remarkable mechanical properties [63].
انظر أيضاً
الهامش
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةBuseck_et_al - ^ Schultz, H.P. (1965). "Topological Organic Chemistry. Polyhedranes and Prismanes". Journal of Organic Chemistry. 30: 1361. doi:10.1021/jo01016a005.
- ^
Osawa, E. (1970). Kagaku. 25: 854.
{{cite journal}}: Missing or empty|title=(help) - ^ Halford, B. (9 October 2006). "The World According to Rick". Chemical & Engineering News. 84 (41): 13.
- ^ Thrower, P.A. (1999). "Editorial". Carbon. 37: 1677. doi:10.1016/S0008-6223(99)00191-8.
- ^ Henson, R.W. "The History of Carbon 60 or Buckminsterfullerene". Retrieved 2010-07-04.
- ^ H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley (1985). "C60: Buckminsterfullerene". Nature. 318: 162–163. doi:10.1038/318162a0.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://machinedesign.com/ContentItem/60618/Anewbuckyballbouncesintotown.aspx
- ^ الكربون
- ^ Kagaku,Vol.25,p.854(1970)
- ^ World of Carbon
- ^ Kagaku,Vol.25,p.854(1970)
- ^ Gary L. Miessler; Donald A. Tarr (2004). Inorganic Chemistry (3 ed.). Pearson Education International. ISBN 0-13-120198-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ D. R. Mitchel; et al. (2001). "The Synthesis of Megatubes: New Dimensions in Carbon Materials". Inorg. Chem. 40: 2751. doi:10.1021/ic000551q.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ N. Sano (2001). "Synthesis of carbon 'onions' in water". Nature (London). 414: 506. doi:10.1038/35107141.
- ^ A. A. Shvartsburg (1999). J. Phys. Chem. 103: 5275.
{{cite journal}}: Missing or empty|title=(help) - ^ Y. Li; et al. (2001). "Structures and stabilities of C60-rings". Chem. Phys. Lett. 335: 524. doi:10.1016/S0009-2614(01)00064-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Bucky's brother -- The boron buckyball makes its début Jade Boyd April 2007 eurekalert.orgLink
- ^ The boron buckyball has an unexpected Th symmetry G. Gopakumar, Nguyen, M. T., Ceulemans, Arnout, Chem. Phys. lett. 450, 175, 2008.[1]
- ^ "Stuffing improves the stability of fullerenelike boron clusters" Prasad, DLVK; Jemmis, E. D.; Phys. Rev. Lett. 100, 165504, 2008.[2]
- ^ The boron buckyball has an unexpected Th symmetry G. Gopakumar, Nguyen, M. T., Ceulemans, Arnout, Chem. Phys. lett. 450, 175, 2008.[3]
- ^ Buckminsterfullerene: Molecule of the Month
- ^ Goldberg Variations Challenge: Juris Meija, Anal. Bioanal. Chem. 2006 (385) 6-7
- ^ V. L. Pushparaj, M. M. Shaijumon, A. Kumar, S. Murugesan, L. Ci, R. Vajtai, R. J. Linhardt, O. Nalamasu and P. M. Ajayan (2007). "Flexible energy storage devices based on nanocomposite paper". Proceedings of the National Academy of Sciences. 104 (34): 13574. doi:10.1073/pnas.0706508104. PMID 17699622.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tegos, G. (2005). "Cationic Fullerenes Are Effective and Selective Antimicrobial Photosensitizers". Chemistry & Biology. 12 (10): 1127–1135. doi:10.1016/j.chembiol.2005.08.014.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ [4][dead link]
- ^ Beavers, Cm; Zuo, T; Duchamp, Jc; Harich, K; Dorn, Hc; Olmstead, Mm; Balch, Al (2006). "Tb3N@C84: an improbable, egg-shaped endohedral fullerene that violates the isolated pentagon rule". Journal of the American Chemical Society. 128 (35): 11352–3. doi:10.1021/ja063636k. PMID 16939248.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Becker, Luann (2007-02-23). "Impact Event at the Permian-Triassic Boundary: Evidence from Extraterrestrial Noble Gases in Fullerenes". Science. 291 (5508): 1530–3. doi:10.1126/science.1057243. PMID 11222855.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ab initio theoretical predictions of C28, C28H4, C28F4, (Ti at C28)H4, and M at C28 (M = Mg, Al, Si, S, Ca, Sc, Ti, Ge, Zr, and Sn), Guo, Ting; Smalley, Richard E.; Scuseria, Gustavo E., 1993.
- ^ Fullerenes in Solutions, Bezmel'nitsyn, V.N.; Eletskiĭ, A.V.; Okun', M.V., Uspekhi Fizicheskikh Nauk, Russian Academy of Sciences, 41 1998.
- ^ Talyzin A.V., Phase transition C60-C60*4C6H6 in liquid benzene.", J. of Phys. Chem, V101, N47, 1997.
- ^ Talyzin A.V., Engstrцm I, C70 in a Benzene, Hexane and Toluene solutions", J. of Phys. Chem, V102, N34, p6477–6481.
- ^ Arndt, M. (1999). "Wave-particle duality of C60". Nature. 401: 680–682. doi:10.1038/44348.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ The radio show can be heard at: http://www.cbc.ca/quirks/archives/05-06/jun17.html
- ^ Moussa F.; et al. (1996). Fullerene Sci. Technol. 4: 21–29.
{{cite journal}}: Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ Moussa F.; et al. (1997). Proc. Electrochem. Soc. 5: 332–336.
{{cite journal}}: Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ Mori T.; et al. (2006). "Preclinical studies on safety of fullerene upon acute oral administration and evaluation for no mutagenesis". Toxicology. 225: 48–54. doi:10.1016/j.tox.2006.05.001.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
Gharbi, Najla (2005). "[60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity". Nano Letters. 5 (12): 2578–2585. doi:10.1021/nl051866b.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kolosnjaj J.; et al. (2007). "13. Toxicity studies of fullerenes and derivatives". In WC Chan (ed.). Bioapplications of nanoparticles. Toronto: Landes Biosciences. pp. 168–180.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Kolosnjaj J, Szwarc H, Moussa F (2007). "Toxicity studies of carbon nanotubes". Adv. Exp. Med. Biol. 620: 181–204. PMID 18217344.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Poland, C. A., Duffin, R., Kinloch, I., Maynard, A., Wallace, W. A. H., Seaton, A.; et al. (2008). "Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study". Nature Nanotechnology. 3: 423. doi:10.1038/nnano.2008.111.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ W. Krätschmer; et al. (1990). "Solid C60: a new form of carbon". Nature. 347: 354. doi:10.1038/347354a0.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ R. C. Haddon; et al. (1991). "Conducting films of C60 and C70 by alkali-metal doping". Nature. 350: 320. doi:10.1038/350320a0.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ O. Gunnarsson (1997). "Superconductivity in fullerides". Rev. Mod. Phys. 69: 575. doi:10.1103/RevModPhys.69.575.
- ^ A. F. Hebard; et al. (1991). "Superconductivity at 18 K in potassium-doped C60". Nature. 350: 600. doi:10.1038/350600a0.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ K. Holczer; et al. (1991). Science. 252: 1154.
{{cite journal}}: Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ M. J. Rosseinsky; et al. (1991). "Superconductivity at 28 K in Rb_{x}C_{60". Phys. Rev. Lett. 66: 2830. doi:10.1103/PhysRevLett.66.2830.
{{cite journal}}: Explicit use of et al. in:|author=(help)} - ^ C. -C. Chen; et al. (1991). "(RbxK1-x)3C60 Superconductors: Formation of a Continuous Series of Solid Solutions". Science. 253: 886. doi:10.1126/science.253.5022.886.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ O. Zhou; et al. (1992). "Compressibility of M3C60 Fullerene Superconductors: Relation Between Tc and Lattice Parameter". Science. 255: 833. doi:10.1126/science.255.5046.833.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ C. M. Brown; et al. (1999). "Pressure dependence of superconductivity in the Na_{2}Rb_{0.5}Cs_{0.5}C_{60} fulleride". Phys. Rev. B. 59: 4439. doi:10.1103/PhysRevB.59.4439.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ A. Y. Ganin; et al. (2008). "Bulk superconductivity at 38 K in a molecular system". Nature. 7: 367. doi:10.1038/nmat2179.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ K. Tanigaki T. W. Ebbesen S. Saito J. Mizuki J. S. Tsai Y. Kubo & S. Kuroshima (1991). "Superconductivity at 33 K in CsxRbyC60". Nature. 352: 222–223. doi:10.1038/352222a0.
- ^ Y. Iwasa; et al. (2003). "Superconductivity, Mott Hubbard states, and molecular orbital order in intercalated fullerides". J. Phys.: Condens. Matter. 15: R495. doi:10.1088/0953-8984/15/13/202.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ O. Gunnarsson (1997). "Superconductivity in fullerides". Rev. Mod. Phys. 69: 575. doi:10.1103/RevModPhys.69.575.
- ^ S. C. Erwin; et al. (1991). "Electronic structure of crystalline K_{6}C_{60". Phys. Rev. Lett. 67: 1610. doi:10.1103/PhysRevLett.67.1610.
{{cite journal}}: Explicit use of et al. in:|author=(help)} - ^ S. C. Erwin; et al. (1993). "Electronic structure of superconducting Ba_{6}C_{60". Phys. Rev. B. 47: 14657. doi:10.1103/PhysRevB.47.14657.
{{cite journal}}: Explicit use of et al. in:|author=(help)} - ^ S. C. Erwin; et al. (1994). Physica C. 199&200: 600.
{{cite journal}}: Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ Y. Iwasa; et al. (2003). "Superconductivity, Mott Hubbard states, and molecular orbital order in intercalated fullerides". J. Phys.: Condens. Matter. 15: R495. doi:10.1088/0953-8984/15/13/202.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ A. Y. Ganin; et al. (2008). "Bulk superconductivity at 38 K in a molecular system". Nature. 7: 367. doi:10.1038/nmat2179.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ J. E. Han; et al. (2003). "Strong Superconductivity with Local Jahn-Teller Phonons in C_{60} Solids". Phys. Rev. Lett. 90: 167006. doi:10.1103/PhysRevLett.90.167006.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ M. Capone; et al. (2002). "Strongly Correlated Superconductivity". Science. 296: 2634. doi:10.1126/science.1071122.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ State molecule of Texas: Link
- ^ Diamonds lose 'world's hardest' title
للاستزادة
- Aldersey-Williams, Hugh (1995). The Most Beautiful Molecule: The Discovery of the Buckyball. John Wiley & Sons. ISBN 0-471-19333-X.
وصلات خارجية
- Pharmacological Studies on Fullerene (C60), a Novel Carbon Allotrope, and Its Derivatives
- Fullerenes in solution: about anomalous temperature dependence of solubility and solid solvates of fullerenes
- Fullerene and nanotube Gallery
- Properties of C60 fullerene
- CBC Radio | Quirks & Quarks | June 17, 2006 at www.cbc.ca
- Buckyball Workshops by Sir Harry Kroto and the Vega Science Trust
- Center for Nanoscale Science and Technology
- Center for Biological and Environmental Nanotechnology
- Dr. Smalley's brief autobiography
- Dr. Smalley's webpage
- Sir Harry Kroto's webpage
- Interview with James R. Heath, discussing the discovery of C60
- Diffraction and Interference with Fullerenes: Wave-particle duality of C60, University of Vienna
- Fullerene-based architectures for quantum computing in Germany and in Great Britain at the QIP IRC
- Computational Chemistry Wiki
- A Spherical Revelation
- C60 3D-view and pdb-file
- Simple model of Fullerene.
- Story on "Buckyeggs" (UC Davis website)
- Stainless Steel Buckminster Fullerenes
- Rhonditic Steel
- Introduction to fullerites
- Introduction to P-SWNT
- Bucky Balls, a short video explaining the structure of C60 by the Vega Science Trust
- Giant Fullerenes, a short video looking at Giant Fullerenes