يوديد السيزيوم
 CsI crystal
| |
 Scintillating CsI crystal
| |
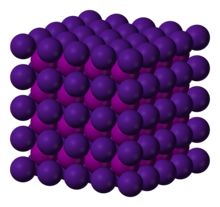
 Crystal structure
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Caesium iodide
| |
| أسماء أخرى
Cesium iodide
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.223 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | CsI |
| كتلة مولية | 259.809 g/mol[2] |
| المظهر | white crystalline solid |
| الكثافة | 4.51 g/cm3[2] |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 848 g/L (25 °C)[2] |
| القابلية المغناطيسية | -82.6·10−6 cm3/mol[4] |
| معامل الانكسار (nD) | 1.9790 (0.3 µm) 1.7873 (0.59 µm) 1.7694 (0.75 µm) 1.7576 (1 µm) 1.7428 (5 µm) 1.7280 (20 µm)[3] |
| البنية | |
| البنية البلورية | CsCl, cP2 |
| الزمرة الفراغية | Pm3m, No. 221[5] |
| ثابت العقد | a = 0.4503 nm |
| هندسة إحداثية |
Cubic (Cs+) Cubic (I−) |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−346.6 kJ/mol[6] |
| Standard molar entropy S |
123.1 J/mol·K[6] |
| سعة الحرارة النوعية، C | 52.8 J/mol·K[6] |
| المخاطر | |
| ن.م.ع. مخطط تصويري |   
|
| ن.م.ع. كلمة الاشارة | Warning |
| H315, H317, H319, H335 | |
| P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |
| نقطة الوميض | Non-flammable |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
2386 mg/kg (oral, rat)[1] |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Caesium fluoride Caesium chloride Caesium bromide Caesium astatide |
كاتيونات أخرى
|
Lithium iodide Sodium iodide Potassium iodide Rubidium iodide Francium iodide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
يوديد السيزيوم Caesium iodide أو cesium iodide (الصيغة الكيميائية CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.[7]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التخليق والبنية
Bulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates.[9]
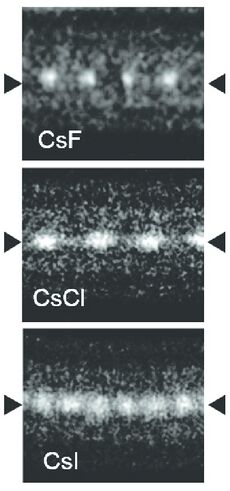
Caesium iodide atomic chains can be grown inside double-wall carbon nanotubes. In such chains I atoms appear brighter than Cs atoms in electron micrographs despite having a smaller mass. This difference was explained by the charge difference between Cs atoms (positive), inner nanotube walls (negative) and I atoms (negative). As a result, Cs atoms are attracted to the walls and vibrate more strongly than I atoms, which are pushed toward the nanotube axis.[8]
الخصائص
| Т (°C) | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (wt%) | 30.9 | 37.2 | 43.2 | 45.9 | 48.6 | 53.3 | 57.3 | 60.7 | 63.6 | 65.9 | 67.7 | 69.2 |
التطبيقات
An important application of caesium iodide crystals, which are scintillators, is electromagnetic calorimetry in experimental particle physics. Pure CsI is a fast and dense scintillating material with relatively low light yield that increases significantly with cooling.[11] It shows two main emission components: one in the near ultraviolet region at the wavelength of 310 nm and one at 460 nm. The drawbacks of CsI are a high temperature gradient and a slight hygroscopicity.
Caesium iodide is used as a beamsplitter in Fourier transform infrared (FTIR) spectrometers. It has a wider transmission range than the more common potassium bromide beamsplitters, working range into the far infrared. However, optical-quality CsI crystals are very soft and hard to cleave or polish. They should also be coated (typically with germanium) and stored in a desiccator, to minimize interaction with atmospheric water vapors.[12]
In addition to image intensifier input phosphors, caesium iodide is often also used in medicine as the scintillating material in flat panel x-ray detectors.[13]
المراجع
- ^ أ ب Cesium iodide. U.S. National Library of Medicine
- ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةb92 - ^ Haynes, p. 10.240
- ^ Haynes, p. 4.132
- ^ Huang, Tzuen-Luh; Ruoff, Arthur L. (1984). "Equation of state and high-pressure phase transition of CsI". Physical Review B. 29 (2): 1112. Bibcode:1984PhRvB..29.1112H. doi:10.1103/PhysRevB.29.1112.
- ^ أ ب ت Haynes, p. 5.10
- ^ Kowalski, M. P.; Fritz, G. G.; Cruddace, R. G.; Unzicker, A. E.; Swanson, N. (1986). "Quantum efficiency of cesium iodide photocathodes at soft x-ray and extreme ultraviolet wavelengths". Applied Optics. 25 (14): 2440. Bibcode:1986ApOpt..25.2440K. doi:10.1364/AO.25.002440. PMID 18231513.
- ^ أ ب Senga, Ryosuke; Komsa, Hannu-Pekka; Liu, Zheng; Hirose-Takai, Kaori; Krasheninnikov, Arkady V.; Suenaga, Kazu (2014). "Atomic structure and dynamic behaviour of truly one-dimensional ionic chains inside carbon nanotubes". Nature Materials. 13 (11): 1050–4. Bibcode:2014NatMa..13.1050S. doi:10.1038/nmat4069. PMID 25218060.
- ^ Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. Bibcode:1951AcCry...4..487S. doi:10.1107/S0365110X51001641.
- ^ Haynes, p. 5.191
- ^ Mikhailik, V.; Kapustyanyk, V.; Tsybulskyi, V.; Rudyk, V.; Kraus, H. (2015). "Luminescence and scintillation properties of CsI: A potential cryogenic scintillator". Physica Status Solidi B. 252 (4): 804–810. arXiv:1411.6246. Bibcode:2015PSSBR.252..804M. doi:10.1002/pssb.201451464. S2CID 118668972.
- ^ Sun, Da-Wen (2009). Infrared Spectroscopy for Food Quality Analysis and Control. Academic Press. pp. 158–. ISBN 978-0-08-092087-0.
- ^ Lança, Luís; Silva, Augusto (2012). "Digital Radiography Detectors: A Technical Overview" (PDF). Digital Imaging Systems for Plain Radiography. Springer. doi:10.1007/978-1-4614-5067-2_2. hdl:10400.21/1932. ISBN 978-1-4614-5066-5. Archived from the original (PDF) on 2019-01-28. Retrieved 2017-08-28.
المصادر المستخدمة
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1439855110.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 | IF, IF3, IF5, IF7 | Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 | S | ICl, ICl3 | Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | Cr | MnI2 | Fe | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 | AsI3 | Se | IBr | Kr |
| RbI | SrI2 | Y | ZrI4 | Nb | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 | SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | Hf | Ta | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 | TlI | PbI2 | Bi | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||