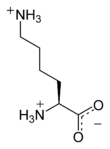
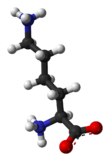
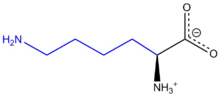
لايسين
| |||
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك
Lysine
| |||
| أسماء أخرى
2,6-Diaminohexanoic acid; 2,6-Diammoniohexanoic acid
| |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.673 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| الخصائص | |||
| الصيغة الجزيئية | C6H14N2O2 | ||
| كتلة مولية | 146.18 g mol-1 | ||
| قابلية الذوبان في الماء | 1.5kg/L @ 25 °C | ||
| علم الأدوية | |||
| B05XB03 (WHO) | |||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
لايسين Lysine هو حمض اميني ينتمي إلى جانب ارجينين وهيستيدين إلى مجموعة الأحماض الأمينية القاعدية, او ما تدعى بسداسية الكربون. حيث تملك جزءا قاعديا يتمثل بحالة ليسين بمجموعة امينية حرة في السلسلة الجانبية، تؤدي إلى تفاعلها كقاعدة.
التخليق الحيوي
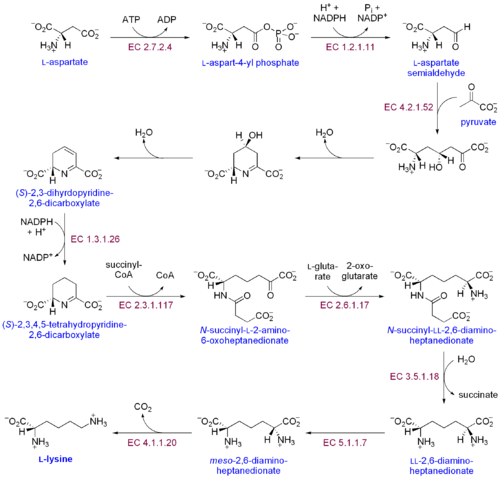
اللايسين هو من الأحماض الأمينية الأساسية بالنسبة للإنسان. ولإنه لا يتشكل في الجسم يجب أن يتم التزود به عن طريق الغذاء حيث يحتاج الأنسان البالغ يوميا ما يقارب من 14 ميلليغرام من الليسين لكل كيلوغرام من وزن الجسم. الاطفال دون سن العاشرة بحاجة ل 44 ميلليغرام لكل كيلوغرام من وزن الجسم يوميا. In plants and most bacteria, it is synthesized from aspartic acid (aspartate):[1]
- L-aspartate is first converted to L-aspartyl-4-phosphate by aspartokinase (or aspartate kinase). ATP is needed as an energy source for this step.
- β-Aspartate semialdehyde dehydrogenase converts this into β-aspartyl-4-semialdehyde (or β-aspartate-4-semialdehyde). Energy from NADPH is used in this conversion.
- 4-hydroxy-tetrahydrodipicolinate synthase adds a pyruvate group to the β-aspartyl-4-semialdehyde, and a water molecule is removed. This causes cyclization and gives rise to (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate.
- This product is reduced to 2,3,4,5-tetrahydrodipicolinate (or Δ1-piperidine-2,6-dicarboxylate, in the figure: (S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate) by 4-hydroxy-tetrahydrodipicolinate reductase. This reaction consumes an NADPH molecule and releases a second water molecule.
- Tetrahydrodipicolinate N-acetyltransferase opens this ring and gives rise to N-succinyl-L-2-amino-6-oxoheptanedionate (or N-acyl-2-amino-6-oxopimelate). Two water molecules and one acyl-CoA (succinyl-CoA) enzyme are used in this reaction.
- N-succinyl-L-2-amino-6-oxoheptanedionate is converted into N-succinyl-LL-2,6-diaminoheptanedionate (N-acyl-2,6-diaminopimelate). This reaction is catalyzed by the enzyme succinyl diaminopimelate aminotransferase. A glutamic acid molecule is used in this reaction and an oxoacid is produced as a byproduct.
- N-succinyl-LL-2,6-diaminoheptanedionate (N-acyl-2,6-diaminopimelate)is converted into LL-2,6-diaminoheptanedionate (L,L-2,6-diaminopimelate) by succinyl diaminopimelate desuccinylase (acyldiaminopimelate deacylase). A water molecule is consumed in this reaction and a succinate is produced a byproduct.
- LL-2,6-diaminoheptanedionate is converted by diaminopimelate epimerase into meso-2,6-diamino-heptanedionate (meso-2,6-diaminopimelate).
- Finally, meso-2,6-diamino-heptanedionate is converted into L-lysine by diaminopimelate decarboxylase.
Enzymes involved in this biosynthesis include:[1]
- Aspartokinase
- Aspartate-semialdehyde dehydrogenase
- 4-hydroxy-tetrahydrodipicolinate synthase
- 4-hydroxy-tetrahydrodipicolinate reductase
- 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase
- Succinyldiaminopimelate transaminase
- Succinyl-diaminopimelate desuccinylase
- Diaminopimelate epimerase
- Diaminopimelate decarboxylase.
It is worth noting, however, that in fungi, euglenoids and some prokaryotes lysine is synthesized via the alpha-aminoadipate pathway.
الأيض
Lysine is metabolised in mammals to give acetyl-CoA, via an initial transamination with α-ketoglutarate. The bacterial degradation of lysine yields cadaverine by decarboxylation.
Allysine is a derivative of lysine, used in the production of elastin and collagen. It is produced by the actions of the enzyme lysyl oxidase on lysine in the extracellular matrix and is essential in the crosslink formation that stabilizes collagen and elastin.
المتطلبات
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For lysine, for adults 19 years and older, 38 mg/kg body weight/day.[2]
التخليق
المصادر الغذائية
الجبنة الپارميزان، والسمك ولحم الخنزير واللحم البقري، فول الصويا، العدس والفول السوداني تحتوي على كمية كبيرة نسبيا من الليسين.
nutritional requirement per day, in milligrams of lysine per kilogram of body weight, is: infants (3–4 months) 103 mg/kg, children (2 years) 64 mg/kg, older children (10–12 years) 44 to 60 mg/kg, adults 12 mg/kg.[3] For a 70 kg adult, 12 milligrams of lysine per kilogram of body weight is 0.84 grams of lysine. Recommendations for adults have been revised upwards to 30 mg/kg.[4]
Good sources of lysine are high-protein foods such as eggs, meat (specifically red meat, lamb, pork, and poultry), soy, beans and peas, cheese (particularly Parmesan), and certain fish (such as cod and sardines).[5]
Lysine is the limiting amino acid (the essential amino acid found in the smallest quantity in the particular foodstuff) in most cereal grains, but is plentiful in most pulses (legumes).[6] A vegetarian or low animal protein diet can be adequate for protein, including lysine, if it includes both cereal grains and legumes, but there is no need for the two food groups to be consumed in the same meals.
A food is considered to have sufficient lysine if it has at least 51 mg of lysine per gram of protein (so that the protein is 5.1% lysine).[7] Foods containing significant proportions of lysine include:
| الغذاء | Lysine (% of protein) |
|---|---|
| السمك | 9.19% |
| اللحم البقري, ground, 90% lean/10% fat, cooked | 8.31% |
| Chicken, roasting, meat and skin, cooked, roasted | 8.11% |
| Azuki bean (adzuki beans), mature seeds, raw | 7.53% |
| Milk, non-fat | 7.48% |
| Soybean, mature seeds, raw | 7.42% |
| Egg, whole, raw | 7.27% |
| Pea, split, mature seeds, raw | 7.22% |
| Lentil, pink, raw | 6.97% |
| Kidney bean, mature seeds, raw | 6.87% |
| Chickpea, (garbanzo beans, Bengal gram), mature seeds, raw | 6.69% |
| Navy bean, mature seeds, raw | 5.73% |
الخصائص
L-Lysine plays a major role in calcium absorption; building muscle protein; recovering from surgery or sports injuries; and the body's production of hormones, enzymes, and antibodies.
التعديلات
انتباه: يتأثر ليسين سلبا في بيئة حارة وجافة كالتحميص على سبيل المثال.
مهام
يتم التحكم بالحمض الأميني لايسين غالباً عبر الترجمة الوراثية. وهذا قد يؤدي إلى الاحتفاظ بحالة الشحن (methylierung أحادي أو ثنائي) أو التخلص منها عبر (acetylierung
في كولاجين تم العثور على لايسين معدل وهو هيدروكسي ليسين بمجموعة OH في السلسلة الجانبية.
وصلات خارجية
- Lysine biosynthesis (early stages), Lysine biosynthesis (later stages), and Lysine catabolism at Queen Mary, University of London
- Computational Chemistry Wiki at compchemwiki.org
- L-Lysine at PDRhealth.com
انظر أيضاً
الهامش
- ^ أ ب ت "MetaCyc: L-lysine biosynthesis I".
- ^ Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768.
- ^ United Nations Food; Agriculture Organization : Agriculture and Consumer Protection. "Energy and protein requirements: 5.6 Requirements for essential amino acids". Retrieved 2010-10-10.
{{cite web}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ FAO/WHO/UNU (2007). "PROTEIN AND AMINO ACID REQUIREMENTS IN HUMAN NUTRITION" (PDF). WHO Press., page 150-152
- ^ University of Maryland Medical Center. "Lysine". Retrieved 2009-12-30.
- ^ Young VR, Pellett PL (1994). "Plant proteins in relation to human protein and amino acid nutrition" (PDF). American Journal of Clinical Nutrition. 59 (5 Suppl): 1203S–1212S. PMID 8172124.
- ^ Institute of Medicine of the National Academies. "Dietary Reference Intakes for Macronutrients". p. 589. Retrieved 2017-10-29.
المصادر
- Much of the information in this article has been translated from German Wikipedia.
- قالب:RubberBible83rd
- CS1 errors: unsupported parameter
- ECHA InfoCard ID from Wikidata
- Articles with changed KEGG identifier
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- Articles that show a Medicine navs template
- Proteinogenic amino acids
- Ketogenic amino acids
- أحماض أمينية قاعدية
- أحماض أمينية أساسية
- Diamines