فوسفيد الغاليوم
 GaP ingots (impure)
| |
 GaP wafer (electronic device quality)
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Gallium phosphide
| |
| أسماء أخرى
Gallium(III) phosphide
gallanylidynephosphane | |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.858 |
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | GaP |
| كتلة مولية | 100.697 g/mol[1] |
| المظهر | pale orange solid |
| الرائحة | odorless |
| الكثافة | 4.138 g/cm3[1] |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | insoluble |
| الفجوة الحزمية | 2.24 eV (indirect, 300 K)[2] |
| حركية الإلكترون | 300 cm2/(V·s) (300 K)[2] |
| القابلية المغناطيسية | -13.8×10-6 cgs[2] |
| التوصيل الحراري | 0.752 W/(cm·K) (300 K)[1] |
| معامل الانكسار (nD) | 2.964 (10 µm), 3.209 (775 nm), 3.590 (500 nm), 5.05 (354 nm)[3] |
| البنية | |
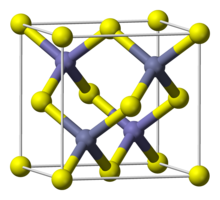
| البنية البلورية | Zinc blende |
| الزمرة الفراغية | T2d-F-43m |
| ثابت العقد | a = 544.95 pm[4] |
| هندسة إحداثية |
Tetrahedral |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−88.0 kJ/mol[5] |
| المخاطر | |
| NFPA 704 (معيـَّن النار) | |
| نقطة الوميض | 110 °C (230 °F; 383 K) |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Gallium nitride Gallium arsenide Gallium antimonide |
كاتيونات أخرى
|
Aluminium phosphide Indium phosphide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
فوسفيد الگاليوم Gallium phosphide (GaP)، فوسفيد الغاليوم، هو مادة شبه موصلة مركبة with an indirect band gap of 2.24 eV at room temperature. Impure polycrystalline material has the appearance of pale orange or grayish pieces. Undoped single crystals are orange, but strongly doped wafers appear darker due to free-carrier absorption. It is odorless and insoluble in water.
GaP has a microhardness of 9450 N/mm2, a Debye temperature of 446 K (173 °C), and a thermal expansion coefficient of 5.3 ×10-6 K−1 at room temperature.[4] Sulfur, silicon or tellurium are used as dopants to produce n-type semiconductors. Zinc is used as a dopant for the p-type semiconductor.
Gallium phosphide has applications in optical systems.[6][7][8] Its static dielectric constant is 11.1 at room temperature.[2] Its refractive index varies between ~3.2 and 5.0 across the visible range, which is higher than in most other semiconducting materials.[3] In its transparent range, its index is higher than almost any other transparent material, including gemstones such as diamond, or non-oxide lenses such as zinc sulfide.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الصمامات الثنائية الباعثة للضوء
Gallium phosphide has been used in the manufacture of low-cost red, orange, and green light-emitting diodes (LEDs) with low to medium brightness since the 1960s. It has a relatively short life at higher current and its lifetime is sensitive to temperature. It is used standalone or together with gallium arsenide phosphide.
Pure GaP LEDs emit green light at a wavelength of 555 nm. Nitrogen-doped GaP emits yellow-green (565 nm) light, zinc oxide doped GaP emits red (700 nm).
Gallium phosphide is transparent for yellow and red light, therefore GaAsP-on-GaP LEDs are more efficient than GaAsP-on-GaAs.
نمو البلورات
At temperatures above ~900 °C, gallium phosphide dissociates and the phosphorus escapes as a gas. In crystal growth from a 1500 °C melt (for LED wafers), this must be prevented by holding the phosphorus in with a blanket of molten boric oxide in inert gas pressure of 10-100 atmospheres. The process is called Liquid Encapsulated Czochralski (LEC) growth, an elaboration of the Czochralski process used for silicon wafers.
انظر أيضاً
مواد ذات صلة
السبائك
الهامش
- ^ أ ب ت Haynes, p. 4.63
- ^ أ ب ت ث Haynes, p. 12.85
- ^ أ ب Haynes, p. 12.156
- ^ أ ب Haynes, p. 12.80
- ^ Haynes, p. 5.20
- ^ Wilson, Dalziel J.; Schneider, Katharina; Hönl, Simon; Anderson, Miles; Baumgartner, Yannick; Czornomaz, Lukas; Kippenberg, Tobias J.; Seidler, Paul (January 2020). "Integrated gallium phosphide nonlinear photonics". Nature Photonics (in الإنجليزية). 14 (1): 57–62. arXiv:1808.03554. doi:10.1038/s41566-019-0537-9. ISSN 1749-4893. S2CID 119357160.
- ^ Cambiasso, Javier; Grinblat, Gustavo; Li, Yi; Rakovich, Aliaksandra; Cortés, Emiliano; Maier, Stefan A. (2017-02-08). "Bridging the Gap between Dielectric Nanophotonics and the Visible Regime with Effectively Lossless Gallium Phosphide Antennas". Nano Letters. 17 (2): 1219–1225. doi:10.1021/acs.nanolett.6b05026. hdl:10044/1/45460. ISSN 1530-6984. PMID 28094990.
- ^ Rivoire, Kelley; Lin, Ziliang; Hatami, Fariba; Masselink, W. Ted; Vučković, Jelena (2009-12-07). "Second harmonic generation in gallium phosphide photonic crystal nanocavities with ultralow continuous wave pump power". Optics Express (in الإنجليزية). 17 (25): 22609–22615. arXiv:0910.4757. doi:10.1364/OE.17.022609. ISSN 1094-4087. PMID 20052186. S2CID 15879811.
مصادر مذكورة
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
