آدرال
 | |
 | |
| البيانات السريرية | |
|---|---|
| الأسماء التجارية | Adderall, Adderall XR, Mydayis |
| أسماء أخرى | Mixed amphetamine salts; MAS |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601234 |
| License data | |
| إحتمالية الإدمان | Moderate[3][4] – high[5][6][7] |
| مسارات الدواء | By mouth, insufflation, rectal, sublingual |
| فئة الدواء | CNS stimulant |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية |
|
| بيانات الحركية الدوائية | |
| التوافر الحيوي | Oral: ~90%[9] |
| المعرفات | |
| رقم CAS | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| (verify) | |
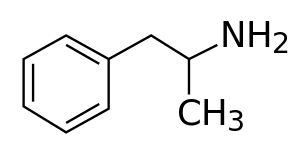
الآدرال (Adderall) والميدايس (Mydayis)[10] هما الاسمان التجاريان [note 2] لدواء مركب يحتوي على أملاح الأمفيتامين. يتكون الخليط من أجزاء متساوية من الأمفيتامين الراسيمي ودكستروأمفيتامين، والذي ينتج نسبة (3:1) بين الدكستروأمفيتامين ولڤوأمفيتامين، وهما المتماكبان المتقابلان للأمفيتامين.[12] كلا المتماكبان من المنشطات، لكنهما يختلفان بدرجة كافية لإعطاء الآدرال ملف تأثيرات مختلف عن تلك الموجودة في الأمفيتامين الراسيمي أو الدكستروأمفيتامين،[1][2] الذي يتم تسويقهما باسم Evekeo دكسدرين/زنزدي على التوالي.[1][13][14] يُستخدم الآدرال في علاج اضطراب قصور الإنتباه وفرط الحركة (ADHD) وخدار. كما يُستخدم بشكل غير مشروع كمُحسِّن للأداء الرياضي، ومُحسِّن للإدراك، ومُثبِّط للشهية، ومادة مبهجة. وهو منشط للجهاز العصبي المركزي من فئة الفنثيلامين.[1]
بالجرعات العلاجية، يسبب الآدرال تأثيرات عاطفية وإدراكية مثل البهجة، وتغير في الدافع الجنسية، وزيادة اليقظة، وتحسين التحكم الإدراكي. عند تناول هذه الجرعات، يسبب الآدرال آثاراً جسدية مثل سرعة رد الفعل ومقاومة التعب وزيادة قوة العضلات. وعلى النقيض من ذلك، يمكن لجرعات أكبر بكثير من الأدرال أن تضعف التحكم الإدراكي، تسبب الانحلال العضلي السريع، تثير نوبات الذعر، أو تحفز الذهان (على سبيل المثال، جنون الارتياب، الوهام، الهلاوس). تختلف الآثار الجانبية بشكل كبير بين الأشخاص، لكن أكثرها شيوعاً يشمل الأرق، جفاف الفم، فقدان الشهية وفقدان الوزن. إن خطر الإصابة بالإدمان أو إدمان المواد ضئيل عند استخدام الآدرال وفقاً للوصفة الطبية وبجرعات يومية منخفضة إلى حد ما، مثل تلك المستخدمة لعلاج قصور الإنتباه وفرط الحركة. ومع ذلك، فإن الاستخدام الروتيني للآدرال بجرعات يومية أكبر يشكل خطراً كبيراً للإدمان أو الاعتياد بسبب التأثيرات التعزيزية الواضحة الموجودة في الجرعات العالية. تكون الجرعات الترفيهية من الآدرال أكبر بكثير من الجرعات العلاجية الموصوفة كما تحمل أيضاً خطراً أكبر بكثير من الآثار الجانبية الخطيرة.[sources 1]
إن اثنين من المتماكبات المتقابلة الأمفيتامينية التي تشكل الآدرال، مثل أقراص/كبسولات آدرال (لڤوأمفيتامين ودكستروأمفيتامين)، تخفف من أعراض اضطراب قصور الإنتباه وفرط الحركة والنوم القهري عن طريق زيادة نشاط الناقل العصبي نورإپينفرين ودوپامين في المخ البشري، والذي ينتج جزئيًا عن تفاعلهما مع مستقبلات الأمين البشري المرتبطة بالأثر 1 (hTAAR1) وناقل الأمين الأحادي الحويصلي 2 (VMAT2) في العصبونات. دكستروأمفيتامين هو منشط أقوى للجهاز العصبي المركزي من اللڤوأمفيتامين، لكن اللڤوأمفيتامين له تأثيرات قلبية وعائية ومحيطية أقوى قليلاً وعمر النصف الحيوي أطول من الدكستروأمفيتامين. أُبلغ عن أن مكون اللڤوأمفيتامين في الآدرال[weasel words] يحسن استجابة العلاج لدى بعض الأشخاص مقارنة بالدكستروأمفيتامين وحده[بحاجة لمصدر]. المادة الفعالة في الآدرال، الأمفيتامين، تشترك في العديد من الخصائص الكيميائية والدوائية مع الأمينات النزرة البشرية، وخاصة الفنثيلامين وN-مثيلفنثيلامين، والأخير هو متزامِر موضعي للأمفيتامين.[sources 2] عام 2022، احتل الآدرال الترتيب 14 كأكثر الأدوية الموصوفة في الولايات المتحدة، بأكثر من 34 مليون وصفة طبية.[34][35]
--->
الاستخدامات
الطبية
Adderall is commonly used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder).[1][16]
قصور الإنتباه وفرط الحركة
الخدار
الأشكال المتوافرة
Adderall is available as immediate-release (IR) tablets and extended-release (XR) capsules.[16][36] Mydayis is only available as an extended-release formulation.[37] Adderall XR is approved to treat ADHD for up to 12 hours in individuals 6 years and older and uses a double-bead formulation. The capsule can be swallowed like a tablet, or it can be opened and the beads sprinkled over applesauce for comparable absorption.[36] Upon ingestion, half of the beads provide immediate administration of medication, while the other half are enveloped in a coating that must dissolve, delaying absorption of its contents. It is designed to provide a therapeutic effect and plasma concentrations identical to taking two doses of Adderall IR four hours apart.[36] Mydayis uses a longer-lasting triple-bead formulation and is approved to treat ADHD for up to 16 hours in individuals 13 years and older.[37] In the United States, the immediate and extended-release formulations of Adderall are both available as generic drugs.[38][39] Generic formulations of Mydayis became available in the US in October 2023.[40]
تحسين الأداء
Adderall has been banned in the National Football League (NFL), Major League Baseball (MLB), National Basketball Association (NBA), and the National Collegiate Athletics Association (NCAA).[41] In leagues such as the NFL, there is a very rigorous process required to obtain an exemption to this rule even when the athlete has been medically prescribed the drug by their physician.[41]
Recreational
Adderall has a high potential for misuse as a recreational drug.[42][43][44] Adderall tablets can either be swallowed, crushed and snorted, or dissolved in water and injected.[45] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[45]
Many postsecondary students have reported using Adderall for study purposes in different parts of the developed world.[44] Among these students, some of the risk factors for misusing ADHD stimulants recreationally include: possessing deviant personality characteristics (i.e., exhibiting delinquent or deviant behavior), inadequate accommodation of disability, basing one's self-worth on external validation, low self-efficacy, earning poor grades, and having an untreated mental health disorder.[44]
موانع الاستخدام
الآثار الجانبية
The adverse side effects of Adderall are many and varied, but the amount of substance consumed is the primary factor in determining the likelihood and severity of side effects.[19][30] Adderall is currently approved for long-term therapeutic use by the USFDA.[19] Recreational use of Adderall generally involves far larger doses and is therefore significantly more dangerous, involving a much greater risk of serious adverse drug effects than dosages used for therapeutic purposes.[30]
الجرعات الزائدة
التداخلات الدوائية
- Monoamine oxidase inhibitors (MAOIs) taken with amphetamine may result in a hypertensive crisis if taken within two weeks after last use of an MAOI type drug.[46]
- Inhibitors of enzymes that directly metabolize amphetamine (particularly CYP2D6 and FMO3) will prolong the elimination of amphetamine and increase drug effects.[46][47][48]
- Serotonergic drugs (such as most antidepressants) co-administered with amphetamine increases the risk of serotonin syndrome.[48]
- Stimulants and antidepressants (sedatives and depressants) may increase (decrease) the drug effects of amphetamine, and vice versa.[46]
- Gastrointestinal and urinary pH affect the absorption and elimination of amphetamine, respectively. Gastrointestinal alkalinizing agents increase the absorption of amphetamine. Urinary alkalinizing agents increase the concentration of non-ionized species, decreasing urinary excretion.[46]
- Proton-pump inhibitors (PPIs) modify the absorption of Adderall XR and Mydayis.[46][48]
- Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 3][52]
- Norepinephrine reuptake inhibitors (NRIs) like atomoxetine prevent norepinephrine release induced by amphetamines and have been found to reduce the stimulant, euphoriant, and sympathomimetic effects of dextroamphetamine in humans.[53][54][55]
علم الأدوية
آلية الحركة
| Compound | NE | DA | 5-HT | Ref | ||
|---|---|---|---|---|---|---|
| Phenethylamine | 10.9 | 39.5 | >10000 | [56][57][58] | ||
| Dextroamphetamine | 6.6–7.2 | 5.8–24.8 | 698–1765 | [59][60] | ||
| Levoamphetamine | 9.5 | 27.7 | ND | [57][58] | ||
| Dextromethamphetamine | 12.3–13.8 | 8.5–24.5 | 736–1291.7 | [59][61] | ||
| Levomethamphetamine | 28.5 | 416 | 4640 | [59] | ||
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs: [62][63] | ||||||
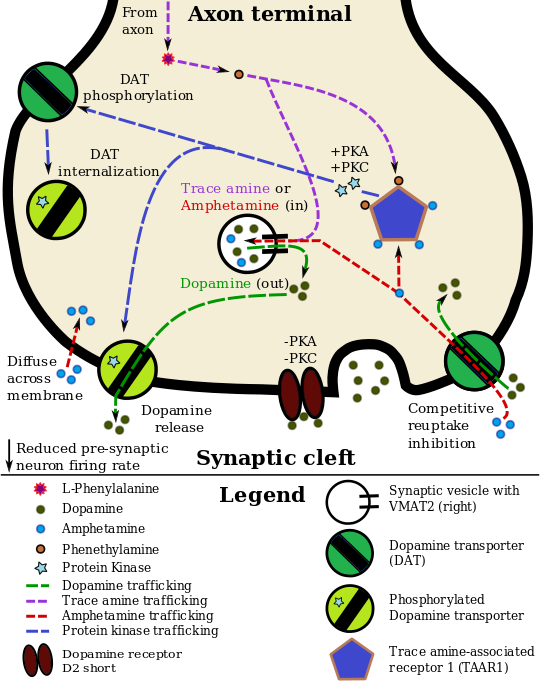
الديناميكية الدوائية للأمفيتامين في العصبو الدوپاميني
|
Amphetamine, the active ingredient of Adderall, works primarily by increasing the activity of the neurotransmitters dopamine and norepinephrine in the brain.[26][69] It also triggers the release of several other hormones (e.g., epinephrine) and neurotransmitters (e.g., serotonin and histamine) as well as the synthesis of certain neuropeptides (e.g., cocaine and amphetamine regulated transcript (CART) peptides).[28][70] Both active ingredients of Adderall, dextroamphetamine and levoamphetamine, bind to the same biological targets,[30][31] but their binding affinities (that is, potency) differ somewhat.[30][31] Dextroamphetamine and levoamphetamine are both potent full agonists (activating compounds) of trace amine-associated receptor 1 (TAAR1) and interact with vesicular monoamine transporter 2 (VMAT2), with dextroamphetamine being the more potent agonist of TAAR1.[31] Consequently, dextroamphetamine produces more CNS stimulation than levoamphetamine;[31][71] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[30] It has been reported that certain children have a better clinical response to levoamphetamine.[32][33]
In the absence of amphetamine, VMAT2 will normally move monoamines (e.g., dopamine, histamine, serotonin, norepinephrine, etc.) from the intracellular fluid of a monoamine neuron into its synaptic vesicles, which store neurotransmitters for later release (via exocytosis) into the synaptic cleft.[28] When amphetamine enters a neuron and interacts with VMAT2, the transporter reverses its direction of transport, thereby releasing stored monoamines inside synaptic vesicles back into the neuron's intracellular fluid.[28] Meanwhile, when amphetamine activates TAAR1, the receptor causes the neuron's cell membrane-bound monoamine transporters (i.e., the dopamine transporter, norepinephrine transporter, or serotonin transporter) to either stop transporting monoamines altogether (via transporter internalization) or transport monoamines out of the neuron;[27] in other words, the reversed membrane transporter will push dopamine, norepinephrine, and serotonin out of the neuron's intracellular fluid and into the synaptic cleft.[27] In summary, by interacting with both VMAT2 and TAAR1, amphetamine releases neurotransmitters from synaptic vesicles (the effect from VMAT2) into the intracellular fluid where they subsequently exit the neuron through the membrane-bound, reversed monoamine transporters (the effect from TAAR1).[27][28]
الحركية الدوائية
الميكروبيومات الدوائية
المركبات الداخلية المتعلقة
التاريخ
The pharmaceutical company Rexar reformulated their popular weight loss drug Obetrol following its mandatory withdrawal from the market in 1973 under the Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act due to the results of the Drug Efficacy Study Implementation (DESI) program (which indicated a lack of efficacy). The new formulation simply replaced the two methamphetamine components with dextroamphetamine and amphetamine components of the same weight (the other two original dextroamphetamine and amphetamine components were preserved), preserved the Obetrol branding, and despite it lacking FDA approval, it still made it onto the market and was marketed and sold by Rexar for many years.
In 1994, Richwood Pharmaceuticals acquired Rexar and began promoting Obetrol as a treatment for ADHD (and later narcolepsy as well), now marketed under the new brand name of Adderall, a contraction of the phrase "A.D.D. for All" intended to convey that "it was meant to be kind of an inclusive thing" for marketing purposes.[72] The FDA cited the company for numerous significant CGMP violations related to Obetrol discovered during routine inspections following the acquisition (including issuing a formal warning letter for the violations), then later issued a second formal warning letter to Richwood Pharmaceuticals specifically due to violations of "the new drug and misbranding provisions of the FD&C Act". Following extended discussions with Richwood Pharmaceuticals regarding the resolution of a large number of issues related to the company's numerous violations of FDA regulations, the FDA formally approved the first Obetrol labeling/sNDA revisions in 1996, including a name change to Adderall and a restoration of its status as an approved drug product.[73][74] In 1997 Richwood Pharmaceuticals was acquired by Shire Pharmaceuticals in a $186 million transaction.[72]
Richwood Pharmaceuticals, which later merged with Shire plc, introduced the current Adderall brand in 1996 as an instant-release tablet.[75] In 2006, Shire agreed to sell rights to the Adderall name for the instant-release form of the medication to Duramed Pharmaceuticals.[76] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 during their acquisition of Barr Pharmaceuticals, including Barr's Duramed division.[77]
The first generic version of Adderall IR was introduced to the market in 2002.[11] Later on, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the extended-release drug beginning in April 2009.[11][78]
التركيبات التجارية
Chemically, Adderall is a mixture of four amphetamine salts; specifically, it is composed of equal parts (by mass) of amphetamine aspartate monohydrate, amphetamine sulfate, dextroamphetamine sulfate, and dextroamphetamine saccharate.[36] This drug mixture has slightly stronger CNS effects than racemic amphetamine due to the higher proportion of dextroamphetamine.[27][30] Adderall is produced as both an immediate-release (IR) and extended-release (XR) formulation.[11][16][46] اعتبارا من ديسمبر 2013[تحديث], ten different companies produced generic Adderall IR, while Teva Pharmaceutical Industries, Actavis, and Barr Pharmaceuticals manufactured generic Adderall XR.[11] اعتبارا من 2013[تحديث], Shire plc, the company that held the original patent for Adderall and Adderall XR, still manufactured brand name Adderall XR, but not Adderall IR.[11]
مقارنة بتركيبات أخرى
Adderall is one of several formulations of pharmaceutical amphetamine, including singular or mixed enantiomers and as an enantiomer prodrug. The table below compares these medications (based on U.S.-approved forms):
| الدواء | التركيبة | الكتلة المولية [note 4] |
قاعدة الأمفيتامين [note 5] |
قاعدة الأمفيتامين في الجرعات المتساوية |
الجرعات بمحتوى قاعدي متساوي [note 6] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (جم/مول) | (النسبة المئوية) | (جرعة 30 مجم) | ||||||||
| الإجمالي | القاعدة | الإجمالي | الدكسترو- | اللـِڤو- | الدكسترو- | اللـِڤو- | ||||
| كبريتات الدكستروأمفيتامين[80][81] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
22.0 مج
|
—
|
30.0 مج
| |
| كبريتات الأمفيتامين[82] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
11.0 مجم
|
11.0 مجم
|
30.0 مجم
| |
| أديرال | 62.57%
|
47.49%
|
15.08%
|
14.2 مجم
|
4.5 مجم
|
35.2 مجم
| ||||
| 25% | كبريتات الدكستروأمفيتامين[80][81] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
73.38%
|
—
|
|||
| 25% | كبريتات الأمفيتامين [82] | (C9H13N)2•H2SO4 | 368.49
|
270.41
|
73.38%
|
36.69%
|
36.69%
|
|||
| 25% | سكريدات الدكستروأمفيتامين[83] | (C9H13N)2•C6H10O8 | 480.55
|
270.41
|
56.27%
|
56.27%
|
—
|
|||
| 25% | مونوهيدرات أسپارتات الأمفيتامين [84] | (C9H13N)•C4H7NO4•H2O | 286.32
|
135.21
|
47.22%
|
23.61%
|
23.61%
|
|||
| ديميسيلات الليزديكسامفيتامين[85] | C15H25N3O•(CH4O3S)2 | 455.49
|
135.21
|
29.68%
|
29.68%
|
—
|
8.9 mg
|
—
|
74.2 mg
| |
| معلق الأمفيتامين القاعدي[86] | C9H13N | 135.21
|
135.21
|
100%
|
76.19%
|
23.81%
|
22.9 مجم
|
7.1 مجم
|
22.0 مجم
| |
المجتمع والثقافة
الوضع القانوني
- In Canada, amphetamines are in Schedule I of the Controlled Drugs and Substances Act, and can only be obtained by prescription.[87]
- In Japan, the use, production, and import of any medicine containing amphetamines is prohibited.[88]
- In South Korea, amphetamines are prohibited.[89]
- In Taiwan, amphetamines including Adderall are Schedule 2 drugs with a minimum five-year prison term for possession.[90] On the contrary, Ritalin can be legally prescribed as a form of treatment of ADHD.[91]
- In Thailand, amphetamines are classified as Type 1 Narcotics.[92]
- In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in prison and an unlimited fine.[93]
- In the United States, amphetamine is a Schedule II prescription drug, classified as a central nervous system (CNS) stimulant.[8]
- Internationally, amphetamine is in Schedule II of the Convention on Psychotropic Substances.[94][95]
النقص
In February 2023, news organizations began reporting on shortages of Adderall in the United States that have lasted for over five months.[96][97] The Food and Drug Administration first reported the shortage in October 2022.[98] In May 2023, seven months into the shortage, the Food and Drug Administration commissioner Robert Califf stated that "a number of generic drugs are in shortage at any given time because there's not enough profit". He points out that Adderall is a special case because it is a controlled substance and the amount available for prescription is controlled by the Drug Enforcement Administration. He also faults a "tremendous increase in prescribing" due to virtual prescribing and general overprescribing and overdiagnosing, adding that "if only the people that needed these drugs got them, there probably wouldn't be a [stimulant medication] shortage".[99][100] The shortage has continued into 2024.[101][102][103] It has led to the creation and expansion of businesses that outsource the search for Adderall. One company charges $50 per U.S. customer to hire workers in the Philippines or another country to make phone calls to all the pharmacies located near the customer and check whether they have any Adderall. Celebrity endorsements have contributed to the increased demand for Adderall.[104]
الهوامش
- ^ Salts of racemic amphetamine and dextroamphetamine are mixed in a (1:1) ratio to produce this drug. Because the racemate is composed of equal parts dextroamphetamine and levoamphetamine, this drug can also be described as a mixture of the D and (L)-enantiomers of amphetamine in a (3:1) ratio, although none of the components of the mixture are levoamphetamine salts.[1][2]
- ^ The trade name Adderall is used primarily throughout this article because the four-salt composition of the drug makes its nonproprietary name (dextroamphetamine sulfate 25%, dextroamphetamine saccharate 25%, amphetamine sulfate 25%, and amphetamine aspartate 25%) is excessively lengthy.[11] Mydayis is a relatively new trade name that is not commonly used to refer generally to the mixture.[10]
- ^ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[49][50][51] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[51]
- ^ من أجل التوحيد، حُسبت الكتل المولية باستخدام حاسبة الوزن الجزيئي[79] وكانت في حدود 0.01 جم/مول من القيم الصيدلانية المنشورة.
- ^ النسبة المئوية لقاعدة الأمفيتامين = الكتلة الموليةالقاعدية / الكتلة الموليةالإجمالية . النسبة المئوية لقاعدة الأمفيتامين في الأديرال = مجموع النسب المئوية للمكونات / 4.
- ^ dose = (1 / النسبة المئوية لقاعدة الأمفيتامين) &المرات؛ عامل القياس = (الكتلة المولية الإجمالية / الكتلة الموليةالقاعدية) &المرات؛ عامل القياس. تم قياس القيم في هذا العمود على أساس جرعة 30 مجم من كبريتات الدكستروأمفيتامين. نظرًا للاختلافات الدوائية بين هذه الأدوية (على سبيل المثال، الاختلافات في الإطلاق، والامتصاص، والتحويل، والتركيز، والتأثيرات المختلفة للنظائر المتماثلة، ونصف العمر، وما إلى ذلك)، لا ينبغي اعتبار القيم المدرجة جرعات متساوية الفعالية.
- مفتاح الصورة
المصادر
المراجع
- ^ أ ب ت ث ج Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ أ ب Joyce BM, Glaser PE, Gerhardt GA (April 2007). "Adderall produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers". Psychopharmacology. 191 (3): 669–677. doi:10.1007/s00213-006-0550-9. PMID 17031708. S2CID 20283057.
- ^ Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America. 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ^ Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, Döpfner M, Hamilton R, Hollis C, Holtmann M, Hulpke-Wette M, Lecendreux M, Rosenthal E, Rothenberger A, Santosh P, Sergeant J, Simonoff E, Sonuga-Barke E, Wong IC, Zuddas A, Steinhausen HC, Taylor E (January 2011). "European guidelines on managing adverse effects of medication for ADHD". European Child & Adolescent Psychiatry. 20 (1): 17–37. doi:10.1007/s00787-010-0140-6. eISSN 1435-165X. PMC 3012210. PMID 21042924.
- ^ Kociancic T, Reed MD, Findling RL (March 2004). "Evaluation of risks associated with short- and long-term psychostimulant therapy for treatment of ADHD in children". Expert Opinion on Drug Safety. 3 (2): 93–100. doi:10.1517/14740338.3.2.93. eISSN 1744-764X. PMID 15006715. S2CID 31114829.
- ^ Clemow DB, Walker DJ (September 2014). "The potential for misuse and abuse of medications in ADHD: a review". Postgraduate Medicine. 126 (5): 64–81. doi:10.3810/pgm.2014.09.2801. eISSN 1941-9260. PMID 25295651. S2CID 207580823.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةStahl's Essential Psychopharmacology - ^ أ ب Ingersoll, John (July 7, 1971). "Amphetamine, Methamphetamine, and Optical Isomers" (PDF). Federal Register. Bureau of Narcotics and Dangerous Drugs. Archived (PDF) from the original on November 27, 2024. Retrieved November 27, 2024.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةhandbook2022 - ^ أ ب Sagonowsky E (28 August 2017). "Shire launches new ADHD drug Mydayis as it weighs a neuroscience exit". Fierce Pharma. Questex LLC. Archived from the original on 16 December 2019. Retrieved 2 May 2020.
- ^ أ ب ت ث ج ح "National Drug Code Amphetamine Search Results". National Drug Code Directory. United States Food and Drug Administration. Archived from the original on 16 December 2013. Retrieved 16 December 2013.
- ^ Babiskin AH, Zhang X (September 2015). "Application of Physiologically Based Absorption Modeling for Amphetamine Salts Drug Products in Generic Drug Evaluation". Journal of Pharmaceutical Sciences. 104 (9): 3170–3182. doi:10.1002/jps.24474. PMID 25973928.
- ^ "Pharmacology". Evekeo CII (amphetamine sulfate) HCP. Arbor Pharmaceuticals, LLC. Archived from the original on 21 September 2020. Retrieved 2 May 2020.
- ^ "Prescribing Information & Medication Guide" (PDF). Zenzedi (dextroamphetamine sulfate, USP). Arbor Pharmaceuticals LLC. Archived (PDF) from the original on 11 November 2020. Retrieved 2 May 2020.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةLibido - ^ أ ب ت ث "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. Teva Pharmaceuticals USA, Inc. 8 November 2019. Archived from the original on 2 October 2019. Retrieved 22 December 2019.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةMalenka_2009 - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةErgogenics - ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةFDA - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةCochrane - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةStimulant Misuse - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةNHMH_3e-Addiction doses - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةAddiction risk - ^ Stolerman IP (2010). Stolerman IP (ed.). Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. p. 78. ISBN 9783540686989.
- ^ Howell LL, Kimmel HL (January 2008). "Monoamine transporters and psychostimulant addiction". Biochemical Pharmacology. 75 (1): 196–217. doi:10.1016/j.bcp.2007.08.003. PMID 17825265.
- ^ أ ب Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- ^ أ ب ت ث ج ح خ د ذ ر Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468. خطأ استشهاد: وسم
<ref>غير صالح؛ الاسم "Miller" معرف أكثر من مرة بمحتويات مختلفة. - ^ أ ب ت ث ج ح خ Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). ... AMPH release of DA from synapses requires both an action at VMAT2 to release DA to the cytoplasm and a concerted release of DA from the cytoplasm via "reverse transport" through DAT.
خطأ استشهاد: وسم<ref>غير صالح؛ الاسم "E Weihe" معرف أكثر من مرة بمحتويات مختلفة. - ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ أ ب ت ث ج ح خ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةWestfall - ^ أ ب ت ث ج Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ أ ب Anthony E (11 November 2013). Explorations in Child Psychiatry. Springer Science & Business Media. pp. 93–94. ISBN 9781468421279. Archived from the original on 21 May 2016. Retrieved 28 April 2015.
- ^ أ ب Arnold LE (2000). "Methyiphenidate vs. Amphetamine: Comparative review". Journal of Attention Disorders. 3 (4): 200–211. doi:10.1177/108705470000300403. S2CID 15901046.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Dextroamphetamine; Dextroamphetamine Saccharate; Amphetamine; Amphetamine Aspartate Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ أ ب ت ث "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
- ^ أ ب "Mydayis medication guide" (PDF). Mydayis.com (in الإنجليزية). October 2023. Retrieved 6 February 2024.
- ^ "Generic Adderall Availability". Drugs.com (in الإنجليزية). Archived from the original on 28 May 2020. Retrieved 6 February 2020.
- ^ "Generic Adderall XR Availability". Drugs.com (in الإنجليزية). Archived from the original on 6 February 2020. Retrieved 6 February 2020.
- ^ "Mydayis® (mixed-salts of a single-entity amphetamine product) – First-time generic" (PDF). OptumRx (in الإنجليزية). Retrieved 6 February 2023.
- ^ أ ب Moore DL. "Do pro sports leagues have an Adderall problem?". USA TODAY. Archived from the original on 23 November 2014. Retrieved 4 May 2014.
- ^ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. Archived from the original on 1 May 2012. Retrieved 7 May 2012.
- ^ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Archived from the original on 2 May 2012. Retrieved 7 May 2012.
- ^ أ ب ت Abelman DD (6 October 2017). "Mitigating risks of students use of study drugs through understanding motivations for use and applying harm reduction theory: a literature review". Harm Reduction Journal. 14 (1): 68. doi:10.1186/s12954-017-0194-6. ISSN 1477-7517. PMC 5639593. PMID 28985738.
- ^ أ ب "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Archived from the original on 12 March 2013. Retrieved 27 February 2013.
- ^ أ ب ت ث ج ح "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 8–10. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةFMO - ^ أ ب ت "Mydayis Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. June 2017. pp. 1–21. Archived (PDF) from the original on 9 June 2019. Retrieved 8 August 2017.
- ^ Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Rev. Neurother. 8 (4): 611–625. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ^ Sulzer D (February 2011). "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron. 69 (4): 628–649. doi:10.1016/j.neuron.2011.02.010. PMC 3065181. PMID 21338876.
They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- ^ أ ب Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH (June 2002). "The role of zinc ions in reverse transport mediated by monoamine transporters". J. Biol. Chem. 277 (24): 21505–21513. doi:10.1074/jbc.M112265200. PMID 11940571.
The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET). ... Surprisingly, this amphetamine-elicited efflux was markedly enhanced, rather than inhibited, by the addition of 10 μM Zn2+ to the superfusion buffer (Fig. 2 A, open squares). We stress that Zn2+ per se did not affect basal efflux (Fig. 2 A). ... In many brain regions, Zn2+ is stored in synaptic vesicles and co-released together with glutamate; under basal conditions, the extracellular levels of Zn2+ are low (~10 nM; see Refs. 39, 40). Upon neuronal stimulation, however, Zn2+ is co-released with the neurotransmitters and, consequently, the free Zn2+ concentration may transiently reach values that range from 10–20 μM (10) up to 300 μM (11). The concentrations of Zn2+ shown in this study, required for the stimulation of dopamine release (as well as inhibition of uptake), covered this physiologically relevant range, with maximum stimulation occurring at 3–30 μM. It is therefore conceivable that the action of Zn2+ on hDAT does not merely reflect a biochemical peculiarity but that it is physiologically relevant. ... Thus, when Zn2+ is co-released with glutamate, it may greatly augment the efflux of dopamine.
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". J. Am. Acad. Child Adolesc. Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
Although we did not find a sufficient number of studies suitable for a meta-analysis of PEA and ADHD, three studies20,57,58 confirmed that urinary levels of PEA were significantly lower in patients with ADHD compared with controls. ... Administration of D-amphetamine and methylphenidate resulted in a markedly increased urinary excretion of PEA,20,60 suggesting that ADHD treatments normalize PEA levels. ... Similarly, urinary biogenic trace amine PEA levels could be a biomarker for the diagnosis of ADHD,20,57,58 for treatment efficacy,20,60 and associated with symptoms of inattentivenesss.59 ... With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110
- ^ Treuer T, Gau SS, Méndez L, Montgomery W, Monk JA, Altin M, Wu S, Lin CC, Dueñas HJ (April 2013). "A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability". J Child Adolesc Psychopharmacol. 23 (3): 179–193. doi:10.1089/cap.2012.0093. PMC 3696926. PMID 23560600.
- ^ Heal DJ, Smith SL, Findling RL (2012). "ADHD: current and future therapeutics". Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Current Topics in Behavioral Neurosciences. Vol. 9. pp. 361–390. doi:10.1007/7854_2011_125. ISBN 978-3-642-24611-1. PMID 21487953.
Adjunctive therapy with DL-methylphenidate in atomoxetine partial responders has been successful (Wilens et al. 2009), but this also increases the rates of insomnia, irritability and loss of appetite (Hammerness et al. 2009). This combination therapy has not included amphetamine because blockade of NET by atomoxetine prevents entry of amphetamine into presynaptic noradrenergic terminals (Sofuoglu et al. 2009).
- ^ Sofuoglu M, Poling J, Hill K, Kosten T (2009). "Atomoxetine attenuates dextroamphetamine effects in humans". Am J Drug Alcohol Abuse. 35 (6): 412–416. doi:10.3109/00952990903383961. PMC 2796580. PMID 20014909.
- ^ Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL (February 2015). "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence. 147: 1–19. doi:10.1016/j.drugalcdep.2014.12.005. PMC 4297708. PMID 25548026.
- ^ أ ب Forsyth, Andrea N (22 May 2012). "Synthesis and Biological Evaluation of Rigid Analogues of Methamphetamines". ScholarWorks@UNO. Retrieved 4 November 2024.
- ^ أ ب Blough B (July 2008). "Dopamine-releasing agents" (PDF). In Trudell ML, Izenwasser S (eds.). Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. OL 18589888W.
- ^ أ ب ت Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ^ Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW (2013). "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology. 38 (4): 552–562. doi:10.1038/npp.2012.204. PMC 3572453. PMID 23072836.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ Sulzer D, Cragg SJ, Rice ME (August 2016). "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia. 6 (3): 123–148. doi:10.1016/j.baga.2016.02.001. PMC 4850498. PMID 27141430.
Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
- ^ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (July 2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ^ "TAAR1". GenAtlas. University of Paris. 28 January 2012. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)
- ^ Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
- ^ Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةcognition enhancers - ^ "Amphetamine: Biomolecular Interactions and Pathways". PubChem Compound. National Center for Biotechnology Information. Archived from the original on 13 October 2013. Retrieved 13 October 2013.
- ^ Smith RC, Davis JM (June 1977). "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology. 53 (1): 1–12. doi:10.1007/bf00426687. PMID 407607. S2CID 37967136.
- ^ أ ب Schwarz A (14 December 2013). "The Selling of Attention Deficit Disorder". The New York Times. ISSN 0362-4331. Archived from the original on 1 March 2015. Retrieved 22 April 2017.
- ^ "(Collection of internal FDA information pertaining to the topic of Obetrol/Adderall)" (PDF). www.accessdata.fda.gov. US Food and Drug Administration. Archived (PDF) from the original on 17 May 2017. Retrieved 27 April 2017.
- ^ "REGULATORY NEWS: Richwood's Adderall". Health News Daily. 22 February 1996. Archived from the original on 23 May 2016. Retrieved 29 May 2013.
- ^ "APPROVAL LETTER" (PDF). United States Food and Drug Administration. Archived (PDF) from the original on 22 August 2013. Retrieved 30 December 2013.
- ^ "August 2006 News Archives: Barr and Shire Sign Three Agreements". GenericsWeb. Archived from the original on 8 February 2014. Retrieved 30 December 2013.
WOODCLIFF LAKE, N.J., Aug. 14 /PRNewswire-FirstCall/ – Barr Pharmaceuticals, Inc. today announced that its subsidiary Duramed Pharmaceuticals, Inc. and Shire plc have signed a Product Acquisition Agreement for ADDERALL(R) (immediate-release mixed amphetamine salts) tablets and a Product Development Agreement for six proprietary products, and that its subsidiary Barr Laboratories, Inc. (Barr) has signed a Settlement and License Agreement relating to the resolution of two pending patent cases involving Shire's ADDERALL XR(R) ...
- ^ "Teva Completes Acquisition of Barr". Drugs.com. Archived from the original on 8 March 2012. Retrieved 31 October 2011.
- ^ "Teva sells 1st generic of Adderall XL in US". Forbes Magazine. Associated Press. 2 April 2009. Archived from the original on 9 April 2009. Retrieved 22 April 2009.
- ^ "Molecular Weight Calculator". Lenntech. Retrieved 19 August 2015.
- ^ أ ب "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ أ ب "D-amphetamine sulfate". Tocris. 2015. Retrieved 19 August 2015.
- ^ أ ب "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ "Vyvanse Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. May 2017. pp. 17–21. Retrieved 10 July 2017.
- ^ "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". 25 April 2017. Archived from the original on 3 April 2011. Retrieved 23 May 2013.
- ^ "Importing or Bringing Medication into Japan for Personal Use". Japan Ministry of Health, Labour and Welfare. Archived from the original on 13 July 2013. Retrieved 13 October 2013.
- ^ P. (25 May 2012). "Moving to Korea brings medical, social changes". The Korean Times. Archived from the original on 3 October 2014. Retrieved 8 February 2013.
- ^ "Caught in the Dragnet". 2 August 2002. Archived from the original on 19 November 2018. Retrieved 18 November 2018.
- ^ "吃「聰明藥」反而變笨?小孩能吃嗎?利他能副作用公開". 28 June 2023.
- ^ "Thailand Law" (PDF). Government of Thailand. Archived from the original (PDF) on 8 March 2014. Retrieved 23 May 2013.
- ^ "Class A, B and C drugs". Home Office, Government of the United Kingdom. Archived from the original on 4 August 2007. Retrieved 23 July 2007.
- ^ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York: United Nations. ISBN 978-92-1-148223-2. Archived (PDF) from the original on 16 October 2013. Retrieved 7 February 2012.
- ^ International Narcotics Control Board. "List of psychotropic substances under international control" (PDF). Vienna: United Nations. Archived from the original (PDF) on 5 December 2005. Retrieved 19 November 2005.
- ^ "The ADHD medication shortage is getting worse. What went wrong?—Neither drugmakers nor the DEA anticipated a sharp rise in ADHD diagnoses during the pandemic. Now an entire class of medications may be in short supply". NBC. 6 February 2023. Retrieved 25 February 2023.
- ^ "Adderall shortage raises questions about widespread dependency on the drug". PBS Newshour. 25 November 2022. Retrieved 25 February 2023.
- ^ Hart B (27 March 2023). "Where's the Urgency on the Adderall Shortage?". Intelligencer (in الإنجليزية الأمريكية). Retrieved 27 March 2023.
- ^ "'Exciting Time': FDA Commissioner Talks AI and Misinformation". WebMD. 31 May 2023.
- ^ "FDA Commissioner Blames Adderall Shortage on Stimulant Overuse, Telehealth, Generics". Anni Layne Rodgers via Additidemag. 2 June 2023.
- ^ "Adderall Shortage Persists in 2024". Pharma Manufacturing. 1 June 2024. Retrieved 12 July 2024.[dead link]
- ^ Smith, Jane (15 May 2024). "Ongoing Adderall Shortage Affects Millions". Health News. Retrieved 12 July 2024.
- ^ "FDA Update on Adderall Shortage". U.S. Food and Drug Administration. 20 June 2024. Retrieved 12 July 2024.
- ^ Maiberg, Emanuel (2024-06-11). "Americans Are Hiring People in the Philippines to Help Them Find Adderall". 404 Media (in الإنجليزية). Retrieved 2024-10-08.
وصلات خارجية
- "Amphetamine". MedlinePlus.
- CS1 الإنجليزية الأمريكية-language sources (en-us)
- Articles with dead external links from February 2025
- Short description is different from Wikidata
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Pages using infobox drug with unknown parameters
- Infobox drug articles with non-default infobox title
- Articles without InChI source
- Infobox drug tracked parameters
- All articles with specifically marked weasel-worded phrases
- Articles with specifically marked weasel-worded phrases from December 2024
- Articles with unsourced statements from December 2024
- Articles with hatnote templates targeting a nonexistent page
- Articles with broken excerpts
- مقالات فيها عبارات متقادمة منذ ديسمبر 2013
- جميع المقالات التي فيها عبارات متقادمة
- آدرال
- 5-HT1A agonists
- Amphetamine
- Anorectics
- Aphrodisiacs
- Attention deficit hyperactivity disorder management
- Combination psychiatric drugs
- Drugs acting on the cardiovascular system
- Drugs acting on the nervous system
- Drugs developed by Takeda Pharmaceutical Company
- Ergogenic aids
- Euphoriants
- Excitatory amino acid reuptake inhibitors
- Narcolepsy
- Nootropics
- Norepinephrine-dopamine releasing agents
- Phenethylamines
- Pro-motivational agents
- Racemic mixtures
- Stimulants
- Substituted amphetamines
- TAAR1 agonists
- VMAT inhibitors
- Wakefulness-promoting agents
- World Anti-Doping Agency prohibited substances