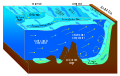
مسرب بارد
| الموائل البحرية |
|---|
 |
| الموائل الساحلية |
| سطح المحيط |
| المحيط المفتوح |
| قاع البحر |
A cold seep (sometimes called a cold vent) is an area of the ocean floor where hydrogen sulfide, methane and other hydrocarbon-rich fluid seepage occurs, often in the form of a brine pool. Cold does not mean that the temperature of the seepage is lower than that of the surrounding sea water. On the contrary, its temperature is often slightly higher.[1] The "cold" is relative to the very warm (at least 60 °C أو 140 °F) conditions of a hydrothermal vent. Cold seeps constitute a biome supporting several endemic species.
Cold seeps develop unique topography over time, where reactions between methane and seawater create carbonate rock formations and reefs. These reactions may also be dependent on bacterial activity. Ikaite, a hydrous calcium carbonate, can be associated with oxidizing methane at cold seeps.
Types

Types of cold seeps can be distinguished according to the depth, as shallow cold seeps and deep cold seeps.[2] Cold seeps can also be distinguished in detail, as follows:
- oil/gas seeps[2]
- gas seeps:[2] methane seeps
- gas hydrate seeps[2]
- brine seeps[2] are forming brine pools
- pockmarks[2]
- mud volcanoes[2]
Formation and ecological succession
Chemosynthetic communities

 Symbiotic vestimentiferan tubeworm Lamellibrachia luymesi from a cold seep at 550 m depth in the Gulf of Mexico. In the sediments around the base are orange bacterial mats of the sulfide-oxidizing bacteria Beggiatoa spp. and empty shells of various clams and snails, which are also common inhabitants of the seeps.[3] |
 Tubeworms, soft corals and chemosynthetic mussels at a seep located 3،000 m (9،800 ft) down on the Florida Escarpment. Eelpouts, a Galatheid crab and an alvinocarid shrimp feed on mussels damaged during a sampling exercise. |
In the Atlantic Ocean

BR – Blake Ridge diapir
BT – Barbados trench
OR – Orenoque sectors
EP – El Pilar sector
NIG – Nigerian slope
GUI – Guiness [ك] area
REG – Regab pockmark.
في البحر المتوسط
The first biological evidence for reduced environments in the Mediterranean Sea was the presence of Lucinidae and Vesicomyidae bivalve shells cored on the top of the Napoli mud volcano (33°43′52″N 24°40′52″E / 33.73111°N 24.68111°E; "Napoli" is only a name of a seamount. It locates south of Crete), located at 1,900 m depth on the Mediterranean Ridge in the subduction zone of the African plate. This was followed by the description of a new Lucinidae bivalve species, Lucinoma kazani, associated with bacterial endosymbionts. In the southeastern Mediterranean, communities of polychaetes and bivalves were also found associated with cold seeps and carbonates near Egypt and the Gaza Strip at depths of 500–800 m, but no living fauna was collected. The first in situ observations of extensive living chemosynthetic communities in the Eastern Mediterranean Sea prompted cooperation between biologists, geochemists, and geologists. During submersible dives, communities comprising large fields of small bivalves (dead and alive), large siboglinid tube worms, isolated or forming dense aggregations, large sponges, and associated endemic fauna were observed in various cold seep habitats associated with carbonate crusts at 1,700–2,000 m depth. Two mud volcano fields were first explored, one along the Mediterranean Ridge, where most of them were partially (Napoli, Milano mud volcanoes) or totally (Urania, Maidstone mud volcanoes) affected by brines, and the other on the Anaximander mounds south of Turkey. The latter area includes the large Amsterdam mud volcano, which is affected by recent mudflows, and the smaller Kazan or Kula mud volcanoes. Gas hydrates have been sampled at the Amsterdam and Kazan mud volcanoes, and high methane levels have been recorded above the seafloor. Several provinces of the Nile deep-sea fan have been explored recently. These include the very active brine seepage named the Menes Caldera in the eastern province between 2,500 m and 3,000 m, the pockmarks in the central area along mid- and lower slopes, and the mud volcanoes of the eastern province, as well as one in the central upper slope (North Alex area) at 500 m depth.[4]
During these first exploratory dives, symbiont-bearing taxa that are similar to those observed on the Olimpi and Anaximander mud fields were sampled and identified. This similarity is not surprising, as most of these taxa were originally described from dredging in the Nile fan.[4] Up to five species of bivalves harboring bacterial symbionts colonized these methane- and sulfide-rich environments. A new species of Siboglinidae polychaete, Lamellibrachia anaximandri, the tubeworm colonizing cold seeps from the Mediterranean ridge to the Nile deep-sea fan, has just been described in 2010.[4][5] Moreover, the study of symbioses revealed associations with chemoautotrophic Bacteria, sulfur oxidizers in Vesicomyidae and Lucinidae bivalves and Siboglinidae tubeworms, and highlighted the exceptional diversity of Bacteria living in symbiosis with small Mytilidae. The Mediterranean seeps appear to represent a rich habitat characterized by megafauna species richness (e.g., gastropods) or the exceptional size of some species such as sponges (Rhizaxinella pyrifera) and crabs (Chaceon mediterraneus), compared with their background counterparts. This contrasts with the low macro- and mega-faunal abundance and diversity of the deep Eastern Mediterranean. Seep communities in the Mediterranean that include endemic chemosynthetic species and associated fauna differ from the other known seep communities in the world at the species level but also by the absence of the large size bivalve genera Calyptogena or Bathymodiolus. The isolation of the Mediterranean seeps from the Atlantic Ocean after the Messinian crisis led to the development of unique communities, which are likely to differ in composition and structure from those in the Atlantic Ocean. Further expeditions involved quantitative sampling of habitats in different areas, from the Mediterranean Ridge to the eastern Nile deep-sea fan.[4] Cold seeps discovered in the Sea of Marmara in 2008[6] have also revealed chemosynthesis-based communities that showed a considerable similarity to the symbiont-bearing fauna of eastern Mediterranean cold seeps.[4]
سجلات أحفورية
Cold seep deposits are found throughout the Phanerozoic rock record, especially in the Late Mesozoic and Cenozoic (see for example Kaim et al., 2008).[7] These fossil cold seeps are characterized by mound-like topography (where preserved), coarsely crystalline carbonates, and abundant mollusks and brachiopods.
انظر أيضاً
الهامش
This article incorporates a public domain work of the United States Government from references[8][9] and CC-BY-2.5 from references[2][10][3][11][12][4][13][14][15] and CC-BY-3.0 text from the reference[16]
- ^ Fujikura, Katsunori; Okutani, Takashi; Maruyama, Tadashi (2008). Sensui chōsasen ga mita shinkai seibutsu : shinkai seibutsu kenkyū no genzai (Deep-sea life: biological observations using research submersibles). Tokai University Press. ISBN 978-4-486-01787-5.
{{cite book}}: Invalid|ref=harv(help) p. 20. - ^ أ ب ت ث ج ح خ د Vanreusel A., De Groote A., Gollner S. & Bright M. (2010). "Ecology and Biogeography of Free-Living Nematodes Associated with Chemosynthetic Environments in the Deep Sea: A Review". PLoS ONE 5(8): e12449. doi:10.1371/journal.pone.0012449.
- ^ أ ب Boetius A. (2005). "Microfauna–Macrofauna Interaction in the Seafloor: Lessons from the Tubeworm". PLoS Biology 3(3): e102. doi:10.1371/journal.pbio.0030102
- ^ أ ب ت ث ج ح Danovaro R., Company J. B., Corinaldesi C., D'Onghia G., Galil B. et al. (2010). "Deep-Sea Biodiversity in the Mediterranean Sea: The Known, the Unknown, and the Unknowable". PLoS ONE 5(8): e11832. doi:10.1371/journal.pone.0011832.
- ^ Southward E., Andersen A., Hourdez S. (submitted 2010). "Lamellibrachia anaximandri n.sp., a new vestimentiferan tubeworm from the Mediterranean (Annelida)". Zoosystema.
- ^ Zitter T. A. C, Henry P., Aloisi G., Delaygue G., Çagatay M. N. et al. (2008). "Cold seeps along the main Marmara Fault in the Sea of Marmara (Turkey)". Deep-Sea Research Part I: Oceanographic Research Papers 55(4): 552–570. doi:10.1016/j.dsr.2008.01.002.
- ^ Kaim A., Jenkins R. & Warén A. (2008). "Provannid and provannid-like gastropods from the Late Cretaceous cold seeps of Hokkaido (Japan) and the fossil record of the Provannidae (Gastropoda: Abyssochrysoidea)". Zoological Journal of the Linnean Society 154(3): 421–436. doi:10.1111/j.1096-3642.2008.00431.x.
- ^ Hsing P.-Y. (19 October 2010). "Gas-powered Circle of Life – Succession in a Deep-sea Ecosystem". NOAA Ocean Explorer | Lophelia II 2010: Oil Seeps and Deep Reefs | 18 October Log. Retrieved 25 January 2011.
- ^ "Gulf of Mexico OCS Oil and Gas Lease Sales: 2007–2012. Western Planning Area Sales 204, 207, 210, 215, and 218. Central Planning Area Sales 205, 206, 208, 213, 216, and 222. Draft Environmental Impact Statement. Volume I: Chapters 1–8 and Appendices" (PDF). Minerals Management Service Gulf of Mexico OCS Region, New Orleans. U.S. Department of the Interior. November 2006. pp. 3–27, 3–31. Archived from the original (PDF) on 26 March 2009.
- ^ Bernardino A. F., Levin L. A., Thurber A. R. & Smith C. R. (2012) "Comparative Composition, Diversity and Trophic Ecology of Sediment Macrofauna at Vents, Seeps and Organic Falls". PLoS ONE 7(4): e33515. doi:10.1371/journal.pone.0033515.
- ^ Olu K., Cordes E. E., Fisher C. R., Brooks J. M., Sibuet M. & Desbruyères D. (2010). "Biogeography and Potential Exchanges Among the Atlantic Equatorial Belt Cold-Seep Faunas". PLoS ONE 5(8): e11967. doi:10.1371/journal.pone.0011967.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةMiloslavich 2011 - ^ Fujikura K., Lindsay D., Kitazato H., Nishida S. & Shirayama Y. (2010). "Marine Biodiversity in Japanese Waters". PLoS ONE 5(8): e11836. doi:10.1371/journal.pone.0011836.
- ^ Gordon D. P., Beaumont J., MacDiarmid A., Robertson D. A. & Ahyong S. T (2010). "Marine Biodiversity of Aotearoa New Zealand". PLoS ONE 5(8): e10905. doi:10.1371/journal.pone.0010905.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةGriffiths 2010 - ^ Oliver G., Rodrigues C & Cunha M. R. (2011). "Chemosymbiotic bivalves from the mud volcanoes of the Gulf of Cadiz, NE Atlantic, with descriptions of new species of Solemyidae, Lucinidae and Vesicomyidae". ZooKeys 113: 1–38. doi:10.3897/ZooKeys.113.1402.
Further reading
- Bright M., Plum C., Riavitz L. A., Nikolov N., Martínez Arbizu P. & Cordes E. E. & Gollner S. (2010). "Epizooic metazoan meiobenthos associated with tubeworm and mussel aggregations from cold seeps of the Northern Gulf of Mexico". Deep-Sea Research Part II: Topical Studies in Oceanography 57(21–23): 1982–1989. doi:10.1016/j.dsr2.2010.05.003.
- German C. R., Ramirez-Llodra E., Baker M. C., Tyler P. A. & the ChEss Scientific Steering Committee (2011). "Deep-Water Chemosynthetic Ecosystem Research during the Census of Marine Life Decade and Beyond: A Proposed Deep-Ocean Road Map". PLoS ONE 6(8): e23259. doi:10.1371/journal.pone.0023259.
- Lloyd K. G., Albert D. B., Biddle J. F., Chanton J. P., Pizarro O. & Teske A. (2010). "Spatial Structure and Activity of Sedimentary Microbial Communities Underlying a Beggiatoa spp. Mat in a Gulf of Mexico Hydrocarbon Seep". PLoS ONE 5(1): e8738. doi:10.1371/journal.pone.0008738.
- Metaxas A. & Kelly N. E. (2010). "Do Larval Supply and Recruitment Vary among Chemosynthetic Environments of the Deep Sea?". PLoS ONE 5(7): e11646. doi:10.1371/journal.pone.0011646.
- Rodríguez E. & Daly M. (2010). "Phylogenetic Relationships among Deep-Sea and Chemosynthetic Sea Anemones: Actinoscyphiidae and Actinostolidae (Actiniaria: Mesomyaria)". PLoS ONE 5(6): e10958. doi:10.1371/journal.pone.0010958.
- Sibuet M. & Olu K. (1998). "Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins". Deep-Sea Research Part II: Topical Studies in Oceanography 45(1–3): 517–567. doi:10.1016/S0967-0645(97)00074-X.
- Vinn, O.; Hryniewicz, K; Little, C.T.S.; Nakrem, H.A. (2014). "A Boreal serpulid fauna from Volgian-Ryazanian (latest Jurassic-earliest Cretaceous) shelf sediments and hydrocarbon seeps from Svalbard". Geodiversitas. 36 (4): 527–540. doi:10.5252/g2014n4a2. Retrieved 9 January 2014.
- Vinn, O.; Kupriyanova, E.K.; Kiel, S. (2013). "Serpulids (Annelida, Polychaeta) at Cretaceous to modern hydrocarbon seeps: Ecological and evolutionary patterns". Palaeogeography, Palaeoclimatology, Palaeoecology. 390: 35–41. doi:10.1016/j.palaeo.2012.08.003. Retrieved 9 January 2014.
وصلات خارجية
- Paul Yancy's vents and seeps page
- Monterey Bay Aquarium Research Institute's seeps page
- ScienceDaily News: Tubeworms in deep sea discovered to have record long life spans