سيانيد الأمونيوم
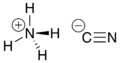
| |
| |
| المُعرِّفات | |
|---|---|
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | NH4CN |
| كتلة مولية | 44.0559 g/mol |
| المظهر | colourless crystalline solid |
| الكثافة | 1.02 g/cm3 |
| نقطة الغليان | |
| قابلية الذوبان في الماء | very soluble |
| قابلية الذوبان | very soluble in alcohol |
| البنية | |
| البنية البلورية | cubic |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Ammonium hydroxide Ammonium azide Ammonium nitrate |
كاتيونات أخرى
|
Sodium cyanide Potassium cyanide |
مركـّبات ذات علاقة
|
Ammonia Hydrogen cyanide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
سيانيد الأمونيوم Ammonium cyanide هو مركب غير عضوي، صيغته NH4CN.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الاستخدامات
Ammonium cyanide is generally used in organic synthesis. Being unstable, it is not shipped or sold commercially.
التحضير
Ammonium cyanide is prepared in solution by bubbling hydrogen cyanide into aqueous ammonia at a low temperature
- HCN + NH3 (aq) → NH4CN (aq)
It may be prepared by the reaction of calcium cyanide and ammonium carbonate:
- Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
In dry state, ammonium cyanide is made by heating a mixture of potassium cyanide or potassium ferrocyanide with ammonium chloride and condensing the vapours into ammonium cyanide crystals:
- KCN + NH4Cl → NH4CN + KCl
التفاعلات
Ammonium cyanide decomposes to ammonia and hydrogen cyanide, often forming a black polymer of hydrogen cyanide:[1]
- NH4CN → NH3 + HCN
It undergoes salt metathesis reaction in solution with a number of metal salts to form metal–cyanide complexes.
Reaction with ketones and aldehydes yield aminonitriles, as in the first step of the Strecker amino acid synthesis:
- NH4CN + CH3COCH3 → (CH3)2C(NH2)CN + H2O
السمية
The solid or its solution is highly toxic. Ingestion can cause death. Exposure to the solid can be harmful as it decomposes to highly toxic hydrogen cyanide and ammonia.
التحليل الكيميائي
Ammonium cyanide may be analyzed by heating the salt and trapping the decomposed products: hydrogen cyanide and ammonia in water at low temperatures. The aqueous solution is analyzed for cyanide ion by silver nitrate titrimetric method or an ion-selective electrode method, and ammonia is measured by titration or electrode technique.
ملاحظات
- ^ Matthews, Clifford N (1991). "Hydrogen cyanide polymerization: A preferred cosmochemical pathway". Bioastronomy: The Search for Extraterrestrial Life—The Exploration Broadens. Lecture Notes in Physics. Vol. 390. pp. 85–87. doi:10.1007/3-540-54752-5_195. ISBN 978-3-540-54752-5.
المراجع
- A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.