ريفاكسيمين
 | |
| البيانات السريرية | |
|---|---|
| مسارات الدواء | عن طريق الفم |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية |
|
| بيانات الحركية الدوائية | |
| التوافر الحيوي | < 0.4% |
| الأيض | عن طريق الكبد |
| Elimination half-life | 6 ساعات |
| الإخراج | Fecal (97%) |
| المعرفات | |
| |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.111.624 |
| Chemical and physical data | |
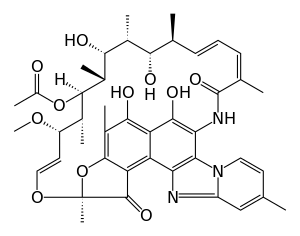
| التركيب | C43H51N3O11 |
| الكتلة المولية | 785.879 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
ريفاكسيمين Rifaximin، هو مضاد حيوي is a semisynthetic, rifamycin-based non-systemic antibiotic، معنى هذا أن مقدار قليل جدا من الدواء سوف يعبر جدار الأمعاء إلى الدورة الدموية مثل جميع الأنواع الأخرى من المضادات الحيوية الفموية. يستخدم لعلاج إسهال السفر والاعتلال الدماغي الكبدي، for which it received orphan drug status from the U.S. Food and Drug Administration in 1998.
الاستخدامات
Rifaximin is licensed by the U.S. Food and Drug Administration to treat traveler's diarrhea caused by E. coli.[1] Clinical trials have shown that rifaximin is highly effective at preventing and treating traveler's diarrhea among travelers to Mexico, with few side effects and low risk of developing antibiotic resistance.[2] [3] It is not effective against Campylobacter jejuni, and there is no evidence of efficacy against Shigella or Salmonella species.
It may be efficacious in relieving chronic functional symptoms of bloating and flatulence that are common in irritable bowel syndrome.[4] There was recently a pilot-study done on the efficacy of rifaximin as a means of treatment for rosacea, according to the study, induced by the co-presence of small intestinal bacterial overgrowth.[5]
In the United States, rifaximin has orphan drug status for the treatment of hepatic encephalopathy.[6] Although high-quality evidence is still lacking, rifaximin appears to be as effective as or more effective than other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster.[7]
A recent study suggests that treatment with rifaximin relieves symptoms for some sufferers of irritable bowel syndrome. [8]
توافر الدواء
يباع ريفاكسيمين في الولايات المتحدة تحت الاسم التجاري إكسيفاكسان Xifaxan من شركة سالكيس للأدوية. ويباع في اوروبا تحت اسم سپيراكسين Spiraxin، زاكسين Zaxine، نورميكس Normix، ريفاكول Rifacol، وكوليدور Colidur وفي الهند تحت اسم ريكسمين RIXMIN.
المصادر
- ^ Xifaxan label informationPDF Retrieved November 15, 2008.
- ^ DuPont, H (2007). "Therapy for and Prevention of Traveler's Diarrhea". Clinical Infectious Diseases. 45 (45 (Suppl 1)): S78–S84. doi:10.1086/518155. PMID 17582576.
- ^ Ruiz J, Mensa L, Pons MJ, Vila J, Gascon J (2008). "Development of Escherichia coli rifaximin-resistant mutants: frequency of selection and stability". The Journal of antimicrobial chemotherapy. 61 (5): 1016–9. doi:10.1093/jac/dkn078. PMID 18325895.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sharara A, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, ElHajj I. (2006). "A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence". Am J Gastroenterol. 101 (2): 326. doi:10.1111/j.1572-0241.2006.00458.x. PMID 16454838.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, Parodi A, Savarino V.
- ^ Wolf, David C. (2007-01-09). "Hepatic Encephalopathy". eMedicine. WebMD. Retrieved 2007-02-15.
- ^ Lawrence KR, Klee JA (2008). "Rifaximin for the treatment of hepatic encephalopathy". Pharmacotherapy. 28 (8): 1019–32. doi:10.1592/phco.28.8.1019. PMID 18657018. Free full text with registration at Medscape.
- ^ Antibiotic May Help Ease Irritable Bowel, Businessweek, January 05, 2011
وصلات خارجية
- Micromedex information on rifaximin
- FDA label approved for Xifaxan (PDF warning)
- Xifaxan (manufacturer's website)
- CS1 errors: unsupported parameter
- Drugs with non-standard legal status
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- Drugboxes which contain changes to watched fields
- أدوية يتيمة
- ريفاكسيمين (مضاد حيوي)