ثلاثي كبريتيد الأنتيمون
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاكs
Antimony(III) sulfide
Diantimony trisulfide | |
أسماء أخرى
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.285 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | S3Sb2 |
| كتلة مولية | 339.72 g mol-1 |
| المظهر | Grey or black orthorhombic crystals (stibnite) |
| الكثافة | 4.562g cm−3 (stibnite)[1] |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 0.00017 g/(100 mL) (18 °C) |
| القابلية المغناطيسية | −86.0·10−6 cm3/mol |
| معامل الانكسار (nD) | 4.046 |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−157.8 kJ/mol |
| سعة الحرارة النوعية، C | 123.32 J/(mol·K) |
| المخاطر | |
| NFPA 704 (معيـَّن النار) | |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
> 2000 mg/kg (rat, oral) |
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |
PEL (المسموح)
|
TWA 0.5 mg/m3 (as Sb)[2] |
REL (الموصى به)
|
TWA 0.5 mg/m3 (as Sb)[2] |
| مركبات ذا علاقة | |
أنيونات أخرى
|
|
كاتيونات أخرى
|
Arsenic trisulfide Bismuth(III) sulfide |
مركـّبات ذات علاقة
|
Antimony pentasulfide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Antimony trisulfide (Sb
2S
3) is found in nature as the crystalline mineral stibnite and the amorphous red mineral (actually a mineraloid)[3] metastibnite.[4] It is manufactured for use in safety matches, military ammunition, explosives and fireworks. It also is used in the production of ruby-colored glass and in plastics as a flame retardant.[5] Historically the stibnite form was used as a grey pigment in paintings produced in the 16th century.[6] In 1817, the dye and fabric chemist, John Mercer discovered the non-stoichiometric compound Antimony Orange (approximate formula Sb
2S
3·Sb
2O
3), the first good orange pigment available for cotton fabric printing.[7]
Antimony trisulfide was also used as the image sensitive photoconductor in vidicon camera tubes. It is a semiconductor with a direct band gap of 1.8–2.5 eV.[بحاجة لمصدر] With suitable doping, p and n type materials can be produced.[8]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Preparation and reactions
Sb
2S
3 can be prepared from the elements at temperature 500–900 °C:[5]
- 2 Sb + 3 S → Sb
2S
3
Sb
2S
3 is precipitated when H
2S is passed through an acidified solution of Sb(III).[9] This reaction has been used as a gravimetric method for determining antimony, bubbling H
2S through a solution of Sb(III) compound in hot HCl deposits an orange form of Sb
2S
3 which turns black under the reaction conditions.[10]
Sb
2S
3 is readily oxidised, reacting vigorously with oxidising agents.[5] It burns in air with a blue flame. It reacts with incandescence with cadmium, magnesium and zinc chlorates. Mixtures of Sb
2S
3 and chlorates may explode.[11]
In the extraction of antimony from antimony ores the alkaline sulfide process is employed where Sb
2S
3 reacts to form thioantimonate(III) salts (also called thioantimonite):[12]
- 3 Na
2S + Sb
2S
3 → 2 Na
3SbS
3
A number of salts containing different thioantimonate(III) ions can be prepared from Sb
2S
3. These include:[13]
- [SbS
3]3−, [SbS
2]−
, [Sb
2S
5]4−, [Sb
4S
9]6−, [Sb
4S
7]2− and [Sb
8S
17]10−
Schlippe's salt, Na
3SbS
4·9H2O, a thioantimonate(V) salt is formed when Sb
2S
3 is boiled with sulfur and sodium hydroxide. The reaction can be represented as:[9]
- Sb
2S
3 + 3 S2− + 2 S → 2 [SbS
4]3−
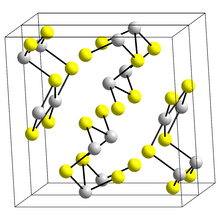
Structure
The structure of the black needle-like form of Sb
2S
3, stibnite, consists of linked ribbons in which antimony atoms are in two different coordination environments, trigonal pyramidal and square pyramidal.[9] Similar ribbons occur in Bi
2S
3 and Sb
2Se
3.[14] The red form, metastibnite, is amorphous. Recent work suggests that there are a number of closely related temperature dependent structures of stibnite which have been termed stibnite (I) the high temperature form, identified previously, stibnite (II) and stibnite (III).[15] Other paper shows that the actual coordination polyhedra of antimony are in fact SbS
7, with (3+4) coordination at the M1 site and (5+2) at the M2 site.قالب:Cln These coordinations consider the presence of secondary bonds. Some of the secondary bonds impart cohesion and are connected with packing.[16]
References
- ^ Haynes, W. M., ed. (2014). CRC Handbook of Chemistry and Physics (95th ed.). Boca Raton, FL: CRC Press. pp. 4–48. ISBN 978-1-4822-0867-2.
- ^ أ ب NIOSH Pocket Guide to Chemical Hazards 0036
- ^ "Metastibnite".
- ^ SUPERGENE METASTIBNITE FROM MINA ALACRAN, PAMPA LARGA, COPIAPO, CHILE, Alan H Clark, THE AMERICAN MINERALOGIST. VOL. 55., 1970
- ^ أ ب ت Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 581–582. ISBN 978-0-08-037941-8.
- ^ Eastaugh, Nicholas (2004). Pigment Compendium: A Dictionary of Historical Pigments. Butterworth-Heinemann. p. 359. ISBN 978-0-7506-5749-5.
- ^ Parnell, Edward A (1886). The life and labours of John Mercer. London: Longmans, Green & Co. p. 23.
- ^ Electrochemistry of Metal Chalcogenides, Mirtat Bouroushian, Springer, 2010
- ^ أ ب ت قالب:Holleman&Wiberg
- ^ A.I. Vogel, (1951), Quantitative Inorganic analysis, (2d edition), Longmans Green and Co
- ^ Hazardous Laboratory Chemicals Disposal Guide, Third Edition, CRC Press, 2003, Margaret-Ann Armour, ISBN 9781566705677
- ^ Anderson, Corby G. (2012). "The metallurgy of antimony". Chemie der Erde - Geochemistry. 72: 3–8. Bibcode:2012ChEG...72....3A. doi:10.1016/j.chemer.2012.04.001. ISSN 0009-2819.
- ^ Inorganic Reactions and Methods, The Formation of Bonds to Group VIB (O, S, Se, Te, Po) Elements (Part 1) (Volume 5) Ed. A.P, Hagen,1991, Wiley-VCH, ISBN 0-471-18658-9
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Kuze S., Du Boulay D., Ishizawa N., Saiki A, Pring A.; (2004), X ray diffraction evidence for a monoclinic form of stibnite, Sb2S3, below 290K; American Mineralogist, 9(89), 1022-1025.
- ^ Kyono, A.; Kimata, M.; Matsuhisa, M.; Miyashita, Y.; Okamoto, K. (2002). "Low-temperature crystal structures of stibnite implying orbital overlap of Sb 5s 2 inert pair electrons". Physics and Chemistry of Minerals. 29 (4): 254–260. Bibcode:2002PCM....29..254K. doi:10.1007/s00269-001-0227-1. S2CID 95067785.
