مسحوق تبييض

التبييض أو القصارة أو مسحوق التبييض Bleach، يشير غلى عدد المواد الكيميائية التي تزيل اللون، المبيض أو المعقم، وعادة ما تتم عن طريق الأكسدة.
عرفت عملية التبييض منذ آلاف السنين،[1] لكن المواد الكيمائية المستخدمة حالياً في عملية التبييض كانت نتيجة لعمل مختلف علماء القرن 18. الكلور هو أساس لمعظم المبيضات الأكثر استخدمااً، على سبيل المثال، محلول تحت كلوريت الصوديوم، الذي يشتهر باسم "المبيض"، وتحت كلوريت الكالسيوم، المكون الأساسي في "مسحوق التبييض". عوامل التبييض المؤكسدة التي لا تحتوي على كلورت يعتمد معظمها على فوق الأكاسيد، مثل فوق أكسيد الهيدروجين، فوق كربونات الصوديوم، وفوق بورات الصوديوم. في الوقت الذي تكون فيه معظم المبيضات عبارة عن عوامل مؤكسدة، فبعضها يكون عوامل إختزال مثل ثاني تيونيت الصوديوم وبورو هيدريد الصوديوم.
المبيضات تستخدم ككيماويات منزلية لتبييض الملابس ولإزالة البقع وكمكطهرات، وخاصة في الحمامات والمطابخ. الكثير من البيضات لديها خصائص قوية كمضادة للجراثيم، وتستخدم للتطهير والتعقيم فتستخدم بالتالي في تعقيم حمامات السباحة للتحكم في الجراثيم، الڤيروسات والطحالب حيث الأماكن التي تتطلب التعقيم. تستخدم أيضاً في الكثير من العمليات الصناعية، وأشهرها في تببيض لب الخشب. يستخدم التبييض أيضاً لإزالة العفن الفطري، قتل الأعشاب الضارة وزيادة عمر الزهور.[2]
التاريخ
The earliest form of bleaching involved spreading fabrics and cloth out in a bleachfield to be whitened by the action of the Sun and water.[1][3] In the 17th century, there was a significant cloth bleaching industry in Western Europe, using alternating alkaline baths (generally lye) and acid baths (such as lactic acid from sour milk, and later diluted sulfuric acid). The whole process lasted up to six months.[1]
Chlorine-based bleaches, which shortened that process from months to hours, were invented in Europe in the late 18th century. Swedish chemist Carl Wilhelm Scheele discovered chlorine in 1774,[1] and in 1785 Savoyard scientist Claude Berthollet recognized that it could be used to bleach fabrics.[1] Berthollet also discovered sodium hypochlorite, which became the first commercial bleach, named Eau de Javel ("Javel water") after the borough of Javel, near Paris, where it was produced.
Scottish chemist and industrialist Charles Tennant proposed in 1798 a solution of calcium hypochlorite as an alternative for Javel water, and patented bleaching powder (solid calcium hypochlorite) in 1799.[1][4] Around 1820, French chemist Antoine Germain Labarraque discovered the disinfecting and deodorizing ability of hypochlorites and was instrumental in popularizing their use for such purpose.[5] His work greatly improved medical practice, public health, and the sanitary conditions in hospitals, slaughterhouses, and all industries dealing with animal products.[6]
Louis Jacques Thénard first produced hydrogen peroxide in 1818 by reacting barium peroxide with nitric acid.[7] Hydrogen peroxide was first used for bleaching in 1882, but did not become commercially important until after 1930.[8] Sodium perborate as a laundry bleach has been used in Europe since the early twentieth century, and became popular in North America in the 1980s.[9]
تحضير مساحيق التبييض
تحضر مساحيق التبييض بإمرار غاز الكلور على الكلس المطفأ. وهناك طريقتان لتحضيرها صناعياً:
تسمى الطريقة الأولى طريقة بخمان، وفيها ينشر الكلس المطفأ على قاع غرف مغلقة ذات أحواض واسعة كي يكون سطح التفاعل كبيراً، ثم يمرر غاز الكلور داخل هذه الغرف فيبدأ التفاعل بسرعة كبيرة في البداية ثم يتباطأ تدريجياً , تحرك كتلة الكلس بوساطة أذرع تتحرك عمودياً، ويتم امتصاص الكلور في 12-14 ساعة.
أما الطريقة الثانية فتدعى طريقة هازنكلفر، وتتم في مفاعلات أسطوانية أفقية (من 3 إلى 8مفاعلات) من معدن مقاوم، متصل بعضها ببعض تسلسلياً وأحدها فوق الآخر، وتجهز كل أسطوانة بغلاف مزدوج يجري فيه ماء بارد للمحافظة على درجة حرارة منخفضة منتظمة (دون الخمسين درجة مئوية)، وفي كل أسطوانة محور متصل بأذرع تعمل خلاطاً. يدفع الكلس المطفأ من الأسطوانة العلوية باستمرار، ويدفع الكلور من الأسطوانة السفلية، وهكذا فإن تيار الكلور الصاعد، يتعاكس مع تيار الكلس المطفأ الهابط فيتم التفاعل.
يراوح محتوى الكلور الفعال في القصارة المستحصل عليها بكلا هاتين الطريقتين بين 35و45 % حسب عدد الأسطوانات وشروط التفاعل ودرجة الحرارة. وتعبأ القصارة الناتجة في أكياس من البولي إثيلين ضمن براميل حديدية أو بلاستيكية.
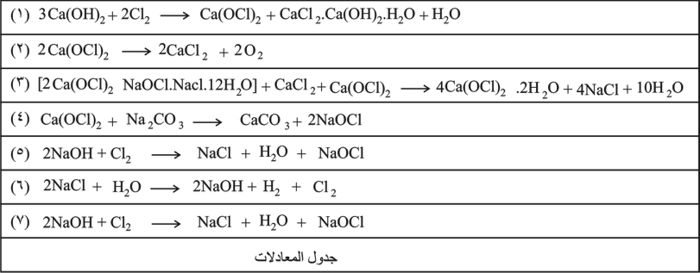
وتعد القصارة من المواد الحاملة للكلور الفعال, إذ تطلقه بسهولة وبالدرجة العادية من الحرارة بتأثير غاز كربون الجو أو الأحماض اللاعضوية، مكونة الملح الكالسيومي, كما تتفاعل مع الماء الأكسجيني فينطلق الأكسجين الفعال، ولذلك تستعمل في تعقيم المياه والمسابح والحمامات والمداجن والمستشفيات كما تستعمل في إزالة ألوان الألياف النباتية (القطن والكتان والقنب والورق). ولدى تخزينها فترة طويلة يحدث تحلل في تركيبها على النحو الآتي: (المعادلة 2 من جدول المعادلات).
تقدر كمية الكلور الفعال في مركبات الهيبو كلوريت كافة (الكالسيوم أو الصوديوم) بمعالجة وزن معين من المركب بكمية زائدة من محلول يود البوتاسيوم المحمض بحمض الخل , ثم معايرة اليود المتحرر بمحلول معروف التركيز من ثيوسلفات الصوديوم.
آلية عمل المبيضات
التبييض
Colors of natural organic materials typically arise from organic pigments, such as beta carotene. Chemical bleaches work in one of two ways:
- An oxidizing bleach works by breaking the chemical bonds that make up the chromophore. This changes the molecule into a different substance that either does not contain a chromophore, or contains a chromophore that does not absorb visible light. This is the mechanism of bleaches based on chlorine but also of oxygen-anions which react through initial nucleophilic attack.[10]
- A reducing bleach works by converting double bonds in the chromophore into single bonds. This eliminates the ability of the chromophore to absorb visible light. This is the mechanism of bleaches based on sulfur dioxide.[11]
Sunlight acts as a bleach through a process leading to similar results: high energy photons of light, often in the violet or ultraviolet range, can disrupt the bonds in the chromophore, rendering the resulting substance colorless. Extended exposure often leads to massive discoloration usually reducing the colors to white and typically very faded blue.[12]
الفعالية ضد الميكروبات
The broad-spectrum effectiveness of most bleaches is due to their general chemical reactivity against organic compounds, rather than the selective inhibitory or toxic actions of antibiotics. They irreversibly denature or destroy many proteins, making them extremely versatile disinfectants.
Hypochlorite bleaches in low concentration were also found to attack bacteria by interfering with heat shock proteins on their walls.[13] According to 2013 Home Hygiene and Health report,[14] using bleach, whether chlorine- or peroxide-based, significantly increases germicidal efficiency of laundry even at low temperatures (30-40 degrees Celsius), which makes it possible to eliminate viruses, bacteria and fungi from variety of clothing in home setting.[15]
أنواع المبيضات
Most industrial and household bleaches belong to three broad classes:
- Chlorine-based bleaches, whose active agent is chlorine, usually from the decomposition of some chlorine compound like hypochlorite or chloramine.
- Peroxide-based bleaches, whose active agent is oxygen, almost always from the decomposition of a peroxide compound like hydrogen peroxide.
- Sulfur dioxide based bleaches, whose active agent is sulfur dioxide, possibly from the decomposition of some oxosulfur anion.
مبيضات الكلور
تحت كلوريت الصوديوم
تحت كلوريت الصوديوم Sodium hypochlorite: ستخدم هيبوكلوريت الصوديوم مادة مطهرة ومزيلة للرائحة في المصانع الغذائية ومشتقاتها، ومعقم للمياه وللأغراض المنزلية، ويستخدم أيضاً مادة قصر وتبييض في مغاسل الملابس. واستخدم إبان الحرب العالمية الأولى في معالجة الجروح كمحلول مستقر متساوي التوتر. وهو عامل تبييض مفيد جداً للقطن والكتان والقنب وعجينة الورق. [16]
يحضر تحت كلوريت الصوديوم بمعالجة محلول هدروكسيد الصوديوم بغاز الكلور: (المعادلة 5 من جدول المعادلات).
وهناك طريقة أخرى لتحضيره أيضاً بالتحليل الكهربائي لمحلول مركز لكلور الصوديوم في خلايا إلكتروليتية لا تحمل رقائق أو أغشية وتعمل بتيار عالي الكثافة في محلول متعادل تقريباً. تصمم الخلايا للعمل عند درجة حرارة منخفضة ولجعل محلول الصودا الكاوية المتشكل عند المهبط على تماس مع الكلور المنطلق عند المصعد. (المعادلتان 6 و7 من جدول المعادلات).
تحت كلوريت الكالسيوم
تحت كلوريت الكالسيوم Calcium hypochlorite: ويمكن تصنيعها بطرائق عدة منها الطريقة التي تستخدم في تصنيع مسحوق التبييض نفسه، ومن ثم فصل هيبوكلوريت الكلسيوم عن المحلول بالتمليح بكلور الصوديوم NaCl وتبخير المحلول فراغياً.
ومنها كذلك كلورة مزيج من هدروكسيد الصوديوم وهدروكسيد الكلسيوم ليتشكل مزيج منهما [2(OCl)2.NaOCl.NaCl.12H2O]، ويتفاعل هذا المزيج مع روبة الكلس المكلورة، ثم يُرشح لإزالة الملح، ويُجفف، فيحتوي الناتج النهائي المستقر 65ـ70% من Ca(OCl)2. وفيما يأتي التفاعل النهائي: (المعادلة 3 من جدول المعادلات).
يتميز تحت كلوريت الكالسيوم بحسنة كبيرة هي كونه لا يتحلل في أثناء التخزين كمسحوق التبييض. وتبلغ نسبة الكلور الفعال فيه ضعف نسبة الكلور الفعال في مسحوق التبييض حيث تبلغ تقريباً 74%. ومن خواصه أنه يذوب في الماء بكثرة وهو غير مسترطب. يستخدم هيبوكلوريت الكلسيوم لتحضير محلول هيبو كلوريت الصوديوم القاصر (ماء جافيل) بمعالجته بمحلول كربونات الصوديوم: (المعادلة 4 من جدول المعادلات).
الكلور
ثاني أكسيد الكلور
مبيضات الپروكسيد
Peroxide-based bleaches are characterized by the peroxide chemical group, namely two oxygen atoms connected by a single bond, (–O–O–). This bond is easily broken, giving rise to very reactive oxygen species, which are the active agents of the bleach.
The main products in this class are:
- پروكسيد الهيدروجين نفسها (H 2O 2). It is used, for example, to bleach wood pulp and hair or to prepare other bleaching agents like the perborates, percarbonates, peracids, etc.
- پركربونات الصوديوم ( Sodium percarbonate ؛ Na 2H 3CO 6), an adduct of hydrogen peroxide and sodium carbonate ("soda ash" or "washing soda", Na 2CO 3). Dissolved in water, it yields a solution of the two products, that combines the degreasing action of the carbonate with the bleaching action of the peroxide.
- أقصى بورات الصوديوم ( sodium perborate ؛ Na 2H 4B 2O 8). Dissolved in water it forms some hydrogen peroxide, but also the perborate anion (B(OOH)(OH)−3) which can perform nucleophilic oxidation.[17]
- Peracetic (peroxoacetic) acid (H 3CC(O)OOH). Generated in situ by some laundry detergents, and also marketed for use as industrial and agricultural disinfection and water treatment.[18]
- Benzoyl peroxide ((C 6H 5COO) 2). It is used in topical medications for acne[19] and to bleach flour.[20]
- Ozone (O 3). While not properly a peroxide, its mechanism of action is similar. It is used in the manufacture of paper products, especially newsprint and white Kraft paper.[21]
- Potassium persulfate (K2 S2O8) and other persulfate salts. It, alongside ammonium and sodium persulfate, are common in hair lightening products.[22]
- Permanganate salts such as Potassium permanganate (KMnO4).
In the food industry, other oxidizing products like bromates are used as flour bleaching and maturing agents.
المبيضات المختزلة
Sodium dithionite (also known as sodium hydrosulfite) is one of the most important reductive bleaching agents. It is a white crystalline powder with a weak sulfurous odor. It can be obtained by reacting sodium bisulfite with zinc
- 2 NaHSO3 + Zn → Na2S2O4 + Zn(OH)2
It is used as such in some industrial dyeing processes to eliminate excess dye, residual oxide, and unintended pigments and for bleaching wood pulp.
Reaction of sodium dithionite with formaldehyde produces Rongalite,
- Na2S2O4 + 2 CH2O + H2O → NaHOCH2SO3 + NaHOCH2SO2
which is used in bleaching wood pulp, cotton, wool, leather and clay.[23]
Photographic bleach
In Reversal processing, residual silver in the emulsion after the first development is reduced to a soluble silver salt using a chemical bleach, most commonly EDTA. A conventional fixer then dissolves the reduced silver but leaving the unexposed silver halide intact. This unexposed halide is then exposed to light or is chemically treated so that a second development produces a positive image. In colour and chromogenic film, this also generates a dye image in proportion to the silver.
Photographic bleaches are also used in black-and-white photography to selectively reduce silver to reduce silver density in negatives or prints. In such cases the bleach composition is typically an acid solution of potassium dichromate.
الوقع البيئي
A Risk Assessment Report (RAR) conducted by the European Union on sodium hypochlorite conducted under Regulation EEC 793/93 concluded that this substance is safe for the environment in all its current, normal uses.[24] This is due to its high reactivity and instability. The disappearance of hypochlorite is practically immediate in the natural aquatic environment, reaching in a short time concentration as low as 10−22 μg/L or less in all emission scenarios. In addition, it was found that while volatile chlorine species may be relevant in some indoor scenarios, they have a negligible impact in open environmental conditions. Further, the role of hypochlorite pollution is assumed as negligible in soils.
Industrial bleaching agents can be sources of concern. For example, the use of elemental chlorine in the bleaching of wood pulp produces organochlorines and persistent organic pollutants, including dioxins. According to an industry group, the use of chlorine dioxide in these processes has reduced the dioxin generation to under detectable levels.[25] However, respiratory risk from chlorine and highly toxic chlorinated byproducts still exists.
A European study conducted in 2008 indicated that sodium hypochlorite and organic chemicals (e.g., surfactants, fragrances) contained in several household cleaning products can react to generate chlorinated volatile organic compounds (VOCs).[26] These chlorinated compounds are emitted during cleaning applications, some of which are toxic and probable human carcinogens. The study showed that indoor air concentrations significantly increase (8–52 times for chloroform and 1–1170 times for carbon tetrachloride, respectively, above baseline quantities in the household) during the use of bleach-containing products. The increase in chlorinated volatile organic compound concentrations was the lowest for plain bleach and the highest for the products in the form of "thick liquid and gel." The significant increases observed in indoor air concentrations of several chlorinated VOCs (especially carbon tetrachloride and chloroform) indicate that the bleach use may be a source that could be important in terms of inhalation exposure to these compounds. While the authors suggested that using these cleaning products may significantly increase the cancer risk,[27] this conclusion appears to be hypothetical:
- The highest level cited for a concentration of carbon tetrachloride (seemingly of highest concern) is 459 micrograms per cubic meter, translating to 0.073 ppm (part per million), or 73 ppb (part per billion). The OSHA-allowable time-weighted average concentration over an eight-hour period is 10 ppm,[28] almost 140 times higher;
- The OSHA highest allowable peak concentration (5-minute exposure for five minutes in a 4-hour period) is 200 ppm,[28] twice as high as the reported highest peak level (from the headspace of a bottle of a sample of bleach plus detergent).
التطهير
Sodium hypochlorite solution, 3–6%, (common household bleach) is typically diluted for safe use when disinfecting surfaces and when used to treat drinking water.[29][30]
A weak solution of 2% household bleach in warm water is typical for sanitizing smooth surfaces prior to the brewing of beer or wine.
US Government regulations (21 CFR 178 Subpart C) allow food processing equipment and food contact surfaces to be sanitized with solutions containing bleach, provided that the solution is allowed to drain adequately before contact with food, and that the solutions do not exceed 200 parts per million (ppm) available chlorine (for example, one tablespoon of typical household bleach containing 5.25% sodium hypochlorite, per gallon of water).
A 1-in-47 dilution of household bleach with water (1 part bleach to 47 parts water) is effective against many bacteria and some viruses in homes.[31] Even "scientific-grade", commercially produced disinfection solutions such as Virocidin-X usually have sodium hypochlorite as their sole active ingredient, though they also contain surfactants (to prevent beading) and fragrances (to conceal the bleach smell).[32]
See Hypochlorous acid for a discussion of the mechanism for disinfectant action.
An oral rinse with a 0.05% dilute solution of household bleach is shown to treat gingivitis.[33]
Diluted sodium hypochlorite at a rate of 2000–1 (0.05% concentration) may represent an efficacious, safe and affordable antimicrobial agent in the prevention and treatment of periodontal disease.
التبييض المؤتمَن على الألوان
Color safe bleach is a chemical that uses hydrogen peroxide as the active ingredient (to help remove stains) rather than sodium hypochlorite or chlorine.[34] It also has chemicals in it that help brighten colors.[35] Hydrogen peroxide is also used for sterilization purposes and water treatment, but its disinfectant capabilities may be limited due to the concentration in the colorsafe bleach solution as compared to other applications.[35]
المخاطر الصحية
The safety of bleaches depends on the compounds present, and their concentration.[36] Generally speaking, the ingestion of bleaches will cause damage to the esophagus and stomach, possibly leading to death. On contact with the skin or eyes, it causes irritation, drying, and potentially burns. Inhalation of bleach fumes can damage the lungs.[36] Personal protective equipment should always be used when using bleach. Bleach should never be mixed with vinegar or other acids, as this will create highly toxic chlorine gas, which can cause severe burns internally and externally.[37][38][39][40] Mixing bleach with ammonia similarly produces toxic chloramine gas, which can burn the lungs.[37][38][40] Mixing bleach with hydrogen peroxide results in an exothermic chemical reaction that releases oxygen, and may cause the contents to splatter and cause skin and eye injury. Heating bleach and boiling it may produce chlorates, a strong oxidizer which may lead to a fire or explosion.
المزاعم الكاذبة بأنه علاج
Miracle Mineral Supplement (MMS), also promoted as "Master Mineral Solution" or "Chlorine Dioxide Solution" or CDS,[41] to evade restrictions by online retail platforms, is a bleach solution that has been fraudulently promoted as a cure-all since 2006.[42] Its main active ingredient is sodium chlorite, which is "activated" with citric acid to form chlorine dioxide. In an attempt to evade health regulations, its inventor, former Scientologist, Jim Humble, formed the Genesis II Church of Health and Healing, a fake religion whose "sacrament" is MMS.[43][44]
During the COVID-19 pandemic advocates of MMS, such as QAnon proponent Jordan Sather and Mark Grenon, who are affiliated with the Genesis II Church, began to suggest this would treat COVID-19.[45][46] Several sources interpreted remarks by U.S. President Trump, in a 23 April 2020 briefing, as promoting this claim,[47][48][49] leading the CDC, scientists, and bleach companies to re-state that bleach is harmful to humans and should not be ingested or injected.[50][49] MSN News quoted Professor Rob Chilcott, a toxicology expert from the University of Hertfordshire, that there is no scientific evidence that injected bleach or disinfectants will affect viral particles, but that injecting bleach would "likely result in significant, irreversible harm and probably a very unpleasant death."[51]
انظر أيضاً
- Calcium hypochlorite
- Hydrogen peroxide
- Milton sterilizing fluid
- تبييض الأسنان
- صودا الغسيل
- كيماويات منلية
المصادر
- ^ أ ب ت ث ج ح "Bleaching". Encyclopaedia Britannica, (9th Edition (1875) and 10th Edition (1902) ed.). Retrieved 2 May 2012.
{{cite encyclopedia}}: CS1 maint: extra punctuation (link) خطأ استشهاد: وسم<ref>غير صالح؛ الاسم "encyc" معرف أكثر من مرة بمحتويات مختلفة. - ^ http://www.rd.com/home/cleaning-organizing/12-smart-ways-to-use-bleach/
- ^ Aspin, Chris (1981). The Cotton Industry. Shire Publications. p. 24. ISBN 978-0-85263-545-2.
- ^ Chisholm 1911.
- ^ Scott, James, transl. (1828). On the disinfecting properties of Labarraque's preparations of chlorine Published by S. Highley.
- ^ Labarraque, Antoine-Germain, Nouvelle biographie générale, volume 28 (1859), columns 323-324.
- ^ L. J. Thénard (1818). "Observations sur des nouvelles combinaisons entre l'oxigène et divers acides". Annales de chimie et de physique. 2nd Series. 8: 306–312.
- ^ Tatjana Topalović (2007). Catalytic Bleaching of Cotton: Molecular and Macroscopic Aspects p 16. Thesis, University of Twente, the Netherlands. ISBN 978-90-365-2454-4. Retrieved 8 May 2012.
- ^ Milne, Neil (1998). "Oxygen bleaching systems in domestic laundry". Journal of Surfactants and Detergents. 1 (2): 253–261. doi:10.1007/s11743-998-0029-z. S2CID 59456079.
- ^ Mayer, Robert J.; Ofial, Armin R. (22 February 2018). "Nucleophilic Reactivities of Bleach Reagents". Organic Letters. 20 (10): 2816–2820. doi:10.1021/acs.orglett.8b00645. PMID 29741385.
- ^ Field, Simon Q (2006). "Ingredients – Bleach". Science Toys. Retrieved 2 March 2006.
- ^ Bloomfield, Louis A (2006). "Sunlight". How Things Work Home Page. Archived from the original on 11 May 2013. Retrieved 23 February 2012.
- ^ Jakob, U.; J. Winter; M. Ilbert; P.C.F. Graf; D. Özcelik (14 November 2008). "Bleach Activates A Redox-Regulated Chaperone by Oxidative Protein Unfolding". Cell. 135 (4): 691–701. doi:10.1016/j.cell.2008.09.024. PMC 2606091. PMID 19013278.
- ^ "| Home Hygiene & Health". www.ifh-homehygiene.org. Retrieved 12 December 2020.
- ^ Ploumanac'h. "Sanitize Laundry for Delicate Clothes". Ploumanac'h (in الإنجليزية). Retrieved 12 December 2020.
- ^ طارق إسماعيل كاخيا. "القصارة". الموسوعة العربية. Retrieved 2014-01-07.
- ^ Douglass F. Taber. "Oxidizing agents: Sodium perborate". Retrieved 7 June 2012.
- ^ V. Namboodiri and A. Garg (2017): "Evaluation of Combined Peracetic acid and UV treatment for Disinfection of Secondary Wastewater Effluent". document EPA/600/R-17/172, National Risk Management Research Laboratory, U.S. Environmental Protection Agency,
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةWHO19th - ^ (2004) "Benzoyl peroxide" FAO Publication FNP 52 Addendum 12.
- ^ "Ozo formulas". Ozone Information. Archived from the original on 15 July 2011. Retrieved 9 January 2009.
- ^ Pang, S.; Fiume, M. Z. (2001). "Final Report on the Safety Assessment of Ammonium, Potassium, and Sodium Persulfate". International Journal of Toxicology (in الإنجليزية). 20 (3_suppl): 7–21. doi:10.1080/10915810152630710. PMID 11766134. S2CID 25763799.
- ^ Herman Harry Szmant (1989). Organic building blocks of the chemical industry. John Wiley and Sons. p. 113. ISBN 978-0-471-85545-3.
- ^ European Union Risk Assessment Report. 2007. Sodium Hypochlorite (CAS No: 7681-52-9; EINECS No: 231-668-3): Final report, November 2007 (Final Approved Version); see Risk Assessment Report on Sodium Hypochlorite, Scientific Committee on Health and Environmental Risks, 12 March 2008.
- ^ "ECF: The Sustainable Technology" (PDF). Alliance for Environmental Technology. Archived from the original (PDF) on 14 April 2008. Retrieved 19 September 2007.
- ^ Odabasi, Mustafa (March 2008). "Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach-Containing Household Products". Environmental Science & Technology. 42 (5): 1445–1451. Bibcode:2008EnST...42.1445O. doi:10.1021/es702355u. PMID 18441786.
- ^ Odabasi, M., Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach- Containing Household Products, Slide presentation (2008)
- ^ أ ب "Chemical Sampling Information: Carbon Tetrachloride". OSHA. 16 June 2004. Retrieved 4 December 2009.
- ^ Dvorak, Glenda (February 2005). "Disinfection" (PDF). Center for Food Security and Public Health. Ames, IA: Center for Food Security and Public Health, Iowa State University. p. 12. Archived from the original (PDF) on 19 June 2010. Retrieved 7 February 2011.
- ^ "Guidelines for the Use of Sanitizers and Disinfectants in Child Care Facilities". Virginia Department of Health. Archived from the original on 14 June 2010. Retrieved 16 March 2010.
- ^ "Everyday Steps and Extra Steps When Someone Is Sick". Center for Disease Control. 11 February 2020.
- ^ "Kam Scientific Inc". Kam Scientific Inc.
- ^ De Nardo, R.; Chiappe, V. N.; Gómez, M.; Romanelli, H.; Slots, J. R. (2012). "Effects of 0.05% sodium hypochlorite oral rinse on supragingival biofilm and gingival inflammation". International Dental Journal. 62 (4): 208–212. doi:10.1111/j.1875-595X.2011.00111.x. PMID 23017003.
- ^ "Dr Laundry - Clorox". 28 October 2015. Archived from the original on 9 June 2011.
- ^ أ ب "Clothes Stain Remover - Pretreat Spray | Clorox®". 27 June 2015.
- ^ أ ب "The clinical toxicology of sodium hypochlorite", Clinical Toxicology 57 (5): 303–311, 2019, doi:, PMID 30689457
- ^ أ ب "Dangers of Mixing Bleach with Cleaners". Washington State Department of Health. Retrieved 12 February 2020.
- ^ أ ب "Some Things Just Don't Mix: Poison Control Tips for Chemicals". Missouri Poison Center. 2 March 2018. Retrieved 12 February 2020.
- ^ "Lesson Learned - Accidental Mixing of Bleach and Acid". Regents of the University of California. Retrieved 12 February 2020.
- ^ أ ب Freedman, Lisa; Mcdonough, Lauren Smith (22 March 2019). "6 Cleaning Products You Should Never, Ever Mix". Good Housekeeping. Retrieved 12 February 2019.
- ^ Loh, John Ming Ren; Shafi, Humaira (24 November 2014). "Kikuchi-Fujimoto disease presenting after consumption of 'Miracle Mineral Solution' (sodium chlorite)". BMJ Case Reports. 2014: bcr2014205832. doi:10.1136/bcr-2014-205832. ISSN 1757-790X. PMC 4244351. PMID 25422331.
- ^ Robbins, Martin (15 September 2010). "The man who encourages the sick and dying to drink industrial bleach". The Guardian (in الإنجليزية البريطانية). ISSN 0261-3077. Retrieved 20 July 2020.
- ^ Food and Drug Administration (12 August 2019). "FDA warns consumers about the dangerous and potentially life threading side effects of Miracle Mineral Solution". fda.gov (in الإنجليزية). Archived from the original on 14 August 2019. Retrieved 16 August 2019.
- ^ Kuruvilla, Carol (22 May 2019). "New Jersey Pastor Has Been Passing Off Bleach As A 'Miracle Cure' In Uganda: Report". HuffPost (in الإنجليزية). Retrieved 25 April 2020.
- ^ Halperin, Dan (in en), QAnon YouTubers Are Telling People to Drink Bleach to Ward Off Coronavirus | Rolling Stone News 1/30/20, https://www.youtube.com/watch?v=QEZpb4an8eM, retrieved on 25 April 2020
- ^ Sommer, Will (10 February 2020). "QAnon Conspiracy Theorists' Magic Cure for Coronavirus Is Drinking Lethal Bleach". The Daily Beast. Archived from the original on 10 February 2020. Retrieved 25 April 2020.
- ^ Pilkington, Ed (24 April 2020). "Revealed: leader of group peddling bleach as coronavirus 'cure' wrote to Trump this week". The Guardian. London. Retrieved 25 April 2020.
- ^ Phillips, Amber (23 April 2020). "Analysis | 3 takeaways from Thursday's White House coronavirus briefing". The Washington Post. Retrieved 25 April 2020.
- ^ أ ب Rogers, Katie; Hauser, Christine; Yuhas, Alan; Haberman, Maggie (24 April 2020). "Trump's Suggestion That Disinfectants Could Be Used to Treat Coronavirus Prompts Aggressive Pushback". The New York Times. Retrieved 25 April 2020.
- ^ "No, don't inject disinfectant: Outcry over Trump's musing". Los Angeles Times. 24 April 2020. Retrieved 25 April 2020.
- ^ Manning, Ellen (24 April 2020). "'Injecting bleach kills!': UK scientists issue warning after Trump coronavirus comments". www.msn.com. Archived from the original on 26 July 2020. Retrieved 25 April 2020.
قراءات إضافية
- طارق إسماعيل كاخيا، مائة مهنة كيميائية بسيطة (دار علاء للنشر، دمشق 2005).
- S. MELLOR, Modern Inorganic Chemistry (Longmans, England 1963).
- S. SHREVE , Chemical Process Industries (McGraw- Hill Book Company 1988).
- Bodkins, Dr. Bailey. Bleach. Philadelphia: Virginia Printing Press, 1995.
- Trotman, E.R. Textile Scouring and Bleaching. London: Charles Griffin & Co., 1968. ISBN 0-85264-067-6.