فلز ضعيف
| الفلزات الأخرى في الجدول الدوري | |||||||
|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| Group → | |||||||
| ↓ Period | |||||||
| 2 | |||||||
| 3 | |||||||
| 4 | |||||||
| 5 | |||||||
| 6 | |||||||
| 7 | |||||||
|
Other metal
Unknown chemical properties
| |||||||
| الرقم الذري color shows state at STP:
أسود=صلب, أخضر=سائل, رمادي=مجهول | |||||||
الفلزات الضعيفة أو الفلزات تحت الإنتقالية العناصر الفلزية هي فلزات المستوى الفرعى p ، وتقع بين أشباه الفلزات والفلزات الإنتقالية. وهى موجبة كهربيا أكثر من الفلزات الإنتقالية ، ولكن أقل من الفلزات القلوية والفلزات القلوية الترابية . ودرجات الذوبان والغليان بصفة عامة أقل من الفلزات الإنتقالية، كما أنها أيضا أقل صلابة.
الفلزات الضعيفة هي: ألومنيوم ، جاليوم ،إنديوم، قصدير، ثاليوم، رصاص، بزموث . العناصر من 113 إلى 116 تم وضعها ضمن هذه المحموعة وتحتوى عناصر تحمل أسماء مؤقتة كالتالى : أنون تريوم ، أنون كواديريوم، أنون بينتينيوم، أنون هيكسينيوم.
<td| 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|
بورون |
كربون | نيتروجين |
أكسجين |
فلور |
ألومنيوم |
سيليكون | فوسفور |
كبريت |
كلور |
جاليوم |
جرمانيوم |
زرنيخ |
سلنيوم |
بروم |
إنديوم |
قصدير |
أنتيمون |
تلوريوم |
يود |
ثاليوم |
رصاص |
بزموث |
بولونيوم |
أستاتين |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
عناصر قابلة للتشغيل
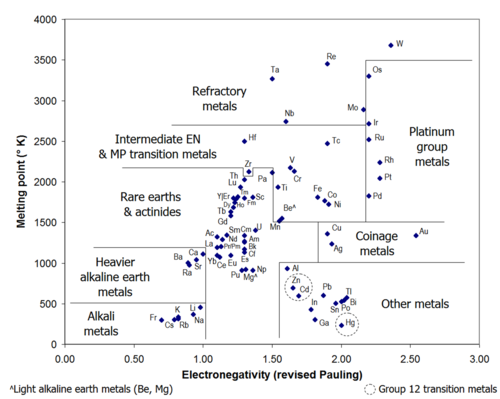
الخصائص
- This section outlines relevant physical and chemical properties of the other metals. For complete profiles, including history, production, specific uses, and biological roles and precautions, see the main article for each element. Abbreviations: MH—Moh's hardness; BCN—bulk coordination number.[n 2]
المجموعة 12
الزنك is a soft metal (MH 2.5) with poor mechanical properties.[7] It has a crystalline structure (BCN 6+6) that is slightly distorted from the ideal. Many zinc compounds are markedly covalent in character.[8] The oxides of zinc in its preferred oxidation state of +2, namely ZnO and Zn(OH)2, are amphoteric;[9] it forms anionic zincates in strongly basic solutions.[10] Zinc forms Zintl phases such as LiZn, NaZn13 and BaZn13.[11] Highly purified zinc, at room temperature, is ductile.[12] It reacts with moist air to form a thin layer of carbonate that prevents further corrosion.[13]
الكادميوم is a soft, ductile metal (MH 2.0) that undergoes substantial deformation, under load, at room temperature.[14] Like zinc, it has a crystalline structure (BCN 6+6) that is slightly distorted from the ideal. The halides of cadmium, with the exception of the fluoride, exhibit a substantially covalent nature.[15] The oxides of cadmium in its preferred oxidation state of +2, namely CdO and CdOH2, are weakly amphoteric; it forms cadmates in strongly basic solutions.[16] Cadmium forms Zintl phases such as LiCd, RbCd13 and CsCd13.[11] When heated in air to a few hundred degrees, cadmium represents a toxicity hazard due to the release of cadmium vapour; when heated to its boiling point in air (just above 1000 K; 725 C; 1340 F; cf steel ~2700 K; 2425 C; 4400 F),[17] the cadmium vapour oxidizes, 'with a reddish-yellow flame, dispersing as an aerosol of potentially lethal CdO particles.'[14] والكادميوم، عدا ذلك، مستقر في الهواء والماء، في الظروف العادية، محمياً بطبقة من أكسيد الكادميوم.
الزئبق is a liquid at room temperature. It has the weakest metallic bonding of all, as indicated by its bonding energy (61 kJ/mol) and melting point (−39 °C) which, together, are the lowest of all the metallic elements.[18][n 3] Solid mercury (MH 1.5)[19] has a distorted crystalline structure,[20] with mixed metallic-covalent bonding,[21] and a BCN of 6. 'All of the [Group 12] metals, but especially mercury, tend to form covalent rather than ionic compounds.'[22] The oxide of mercury in its preferred oxidation state (HgO; +2) is weakly amphoteric, as is the congener sulfide HgS.[23] It forms anionic thiomercurates (such as Na2HgS2 and BaHgS3) in strongly basic solutions.[24][n 4] It forms or is a part of Zintl phases such as NaHg and K8In10Hg.[25] Mercury is a relatively inert metal, showing little oxide formation at room temperature.[26]
المجموعة 13
الألومنيوم in pure form is a soft metal (MH 3.0) with low mechanical strength.[27] It has a close-packed structure (BCN 12) showing some evidence of partially directional bonding.[28][n 5] It has a low melting point (just over half that of steel) and a high thermal conductivity. Its strength is halved at 200 °C, and for many of its alloys is minimal at 300 °C.[30] The latter three properties of aluminium limit its use to situations where fire protection is not required,[31] or necessitate the provision of increased fire protection.[32][n 6] It bonds covalently in most of its compounds;[36] has an amphoteric oxide; and can form anionic aluminates.[10] Aluminium forms Zintl phases such as LiAl, Ca3Al2Sb6, and SrAl2.[37] A thin protective layer of oxide confers a reasonable degree of corrosion resistance.[38] It is susceptible to attack in low pH (<4) and high (> 8.5) pH conditions,[39][n 7] a phenomenon that is generally more pronounced in the case of commercial purity aluminium and aluminium alloys.[45] Given many of these properties and its proximity to the dividing line between metals and nonmetals, aluminium is occasionally classified as a metalloid.[n 8] Despite its shortcomings, it has a good strength-to-weight ratio and excellent ductility; its mechanical strength can be improved considerably with the use of alloying additives; its very high thermal conductivity can be put to good use in heat sinks and heat exchangers;[46] and it has a high electrical conductivity.[n 9] At lower temperatures, aluminium increases its deformation strength (as do most materials) whilst maintaining ductility (as do face-centred cubic metals generally).[48] Chemically, bulk aluminium is a strongly electropositive metal, with a high negative electrode potential.[49][n 10]
الگاليوم is a soft, brittle metal (MH 1.5) that melts at only a few degrees above room temperature.[51] It has an unusual crystalline structure featuring mixed metallic-covalent bonding and low symmetry[51] (BCN 7 i.e. 1+2+2+2).[52] It bonds covalently in most of its compounds,[53] has an amphoteric oxide;[54] and can form anionic gallates.[10] Gallium forms Zintl phases such as Li2Ga7, K3Ga13 and YbGa2.[55] It is slowly oxidized in moist air at ambient conditions; a protective film of oxide prevents further corrosion.[56]
الإنديوم is a soft, highly ductile metal (MH 1.0) with a low tensile strength.[57][58] It has a partially distorted crystalline structure (BCN 4+8) associated with incompletely ionised atoms.[59] The tendency of indium '...to form covalent compounds is one of the more important properties influencing its electrochemical behavior'.[60] The oxides of indium in its preferred oxidation state of +3, namely In2O3 and In(OH)3 are weakly amphoteric; it forms anionic indates in strongly basic solutions.[61] Indium forms Zintl phases such as LiIn, Na2In and Rb2In3.[62] Indium does not oxidize in air at ambient conditions.[58]
الثاليوم is a soft, reactive metal (MH 1.0), so much so that it has no structural uses.[63] It has a close-packed crystalline structure (BCN 6+6) but an abnormally large interatomic distance that has been attributed to partial ionisation of the thallium atoms.[64] Although compounds in the +1 (mostly ionic) oxidation state are the more numerous, thallium has an appreciable chemistry in the +3 (largely covalent) oxidation state, as seen in its chalcogenides and trihalides.[65] It is the only one of the Group 13 elements to react with air at room temperature, slowly forming the amphoteric oxide Tl2O3.[66][67][68] It forms anionic thallates such as Tl3TlO3, Na3Tl(OH)6, NaTlO2, and KTlO2, and is present as the Tl– thallide anion in the compound CsTl.[67][69] Thallium forms Zintl phases, such as Na2Tl, Na2K21Tl19, CsTl and Sr5Tl3H.[70]
المجموعة 14
الصفيح is a soft, exceptionally[71] weak metal (MH 1.5);[n 11] a 1-cm thick rod will bend easily under mild finger pressure.[71] It has an irregularly coordinated crystalline structure (BCN 4+2) associated with incompletely ionised atoms.[59] All of the Group 14 elements form compounds in which they are in the +4, predominately covalent, oxidation state; even in the +2 oxidation state tin generally forms covalent bonds.[73] The oxides of tin in its preferred oxidation state of +2, namely SnO and Sn(OH)2, are amphoteric;[74] it forms stannites in strongly basic solutions.[10] Below 13 °C; 55.4 F tin changes its structure and becomes 'grey tin', which has the same structure as diamond, silicon and germanium (BCN 4). This transformation causes ordinary tin to crumble and disintegrate since, as well as being brittle, grey tin occupies more volume due to having a less efficient crystalline packing structure. Tin forms Zintl phases such as Na4Sn, BaSn, K8Sn25 and Ca31Sn20.[75] It has good corrosion resistance in air on account of forming a thin protective oxide layer. Pure tin has no structural uses.[76] It is used in lead-free solders, and as a hardening agent in alloys of other metals, such as copper, lead, titanium and zinc.[77]
الرصاص is a soft metal (MH 1.5) which, in many cases,[78] is unable to support its own weight.[79] It has a close-packed structure (BCN 12) but an abnormally large inter-atomic distance that has been attributed to partial ionisation of the lead atoms.[64][80] It forms a semi-covalent dioxide PbO2; a covalently bonded sulfide PbS; covalently bonded halides;[81] and a range of covalently bonded organolead compounds such as the lead(II) mercaptan Pb(SC2H5)2, lead tetra-acetate Pb(CH3CO2)4, and the once common, anti-knock additive, tetra-ethyl lead (CH3CH2)4Pb.[82] The oxide of lead in its preferred oxidation state (PbO; +2) is amphoteric;[83] it forms anionic plumbates in strongly basic solutions.[10] Lead forms Zintl phases such as CsPb, Sr31Pb20, La5Pb3N and Yb3Pb20.[84] It has reasonable to good corrosion resistance; in moist air it forms a mixed gray coating of oxide, carbonate and sulfate that hinders further oxidation.[85]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
المجموعة 15
البزموت is a slightly radioactive, soft metal (MH 2.5) that is too brittle for any structural use.[86] It has an open-packed crystalline structure (BCN 3+3) with bonding that is intermediate between metallic and covalent.[87] For a metal, it has exceptionally low electrical and thermal conductivity.[88] Most of the ordinary compounds of bismuth are covalent in nature.[89] The oxide, Bi2O3 is predominately basic but will act as a weak acid in warm, very concentrated KOH.[90] It can also be fused with potassium hydroxide in air, resulting in a brown mass of potassium bismuthate.[91] The solution chemistry of bismuth is characterised by the formation of oxyanions;[92] it forns anionic bismuthates in strongly basic solutions.[93] Bismuth forms Zintl phases such as NaBi,[94] Rb7In4Bi6[95] and Ba11Cd8Bi14.[96] Bailar et al.[97] refer to bismuth as being, 'the least "metallic" metal in its physical properties' given its brittle nature (and possibly) 'the lowest electrical conductivity of all metals.'[n 12]
المجموعة 16
الپولونيوم is radioactive, soft metal with a hardness similar to lead.[99] It has a simple cublc crystalline structure characterised (as determined by electron density calculations) by partially directional bonding,[100] and a BCN of 6. Such a structure ordinarily results in very low ductility and fracture resistance[101] however polonium has been predicted to be a ductile metal.[102] It forms a covalent hydride;[103] its halides are covalent, volatile compounds, resembling those of tellurium.[104] The oxide of polonium in its preferred oxidation state (PoO2; +4) is predominately basic, but amphoteric if dissolved in concentrated aqueous alkali, or fused with potassium hydroxide in air.[105] The yellow polonate(IV) ion PoO2−3 is known in aqueous solutions of low Cl‒ concentration and high pH.[106][n 13] Polonides such as Na2Po, BePo, ZnPo, CdPo and HgPo feature Po2– anions;[108] except for HgPo these are some of the more stable of the polonium compounds.[109][n 14]
تجميعات ذات صلة
B-subgroup metals
Superficially, the B-subgroup metals are the metals in Groups IB to VIB of the periodic table, corresponding to Groups 11 to 16 using current IUPAC nonmenclature. Practically, the group 11 metals (copper, silver and gold) are ordinarily regarded as transition metals (or sometimes as coinage metals, or noble metals) whereas the group 12 metals (zinc, cadmium, and mercury) may or may not be treated as B-subgroup metals depending on if the transition metals are taken to end at group 11 or group 12. The 'B' nomenclature (as in Groups IB, IIB, and so on) was superseded in 1988 but is still occasionally encountered in more recent literature.[111][n 15]
The B-subgroup metals show nonmetallic properties; this is particularly apparent in moving from group 12 to group 16.[113] Although the group 11 metals have normal close-packed metallic structures[114] they show an overlap in chemical properties. In their +1 compounds (the stable state for silver; less so for copper)[115] they are typical B-subgroup metals. In their +2 and +3 states their chemistry is typical of transition metal compounds.[116]
Borderline metals
Parish[117] writes that, 'as anticipated', the borderline metals of groups 13 and 14 have non-standard structures. Gallium, indium, thallium, germanium, and tin are specifically mentioned in this context. The group 12 metals are also noted as having slightly distorted structures; this has been interpreted as evidence of weak directional (i.e. covalent) bonding.[n 16]
Chemically weak metals
Rayner-Canham and Overton[119] use the term chemically weak metals to refer to the metals close to the metal-nonmetal borderline. These metals behave chemically more like the metalloids, particularly with respect to anionic species formation. The nine chemically weak metals identified by them are berylllium, aluminium, zinc, gallium, tin, lead, antimony, bismuth, and polonium.[n 17]
الفلزات الثقيلة (بنقاط انصهار منخفضة)
Van Wert[121] grouped the periodic table metals into a. the light metals; b. the heavy brittle metals of high melting point, c. the heavy ductile metals of high melting point; d. the heavy metals of low melting point (Zn, Cd, Hg; Ga, In, Tl; Ge, Sn; As, Sb, Bi; and Po), and e. the strong, electropositive metals. Britton, Abbatiello and Robins[122] speak of 'the soft, low melting point, heavy metals in columns lIB, IlIA, IVA, and VA of the periodic table, namely Zn, Cd, Hg; Al, Ga, In, Tl; [Si], Ge, Sn, Pb; and Bi. The Sargent-Welch Chart of the Elements groups the metals into: light metals, the lanthanide series; the actinide series; heavy metals (brittle); heavy metals (ductile); and heavy metals (low melting point): Zn, Cd, Hg, [Cn]; Al, Ga, In, Tl; Ge, Sn, Pb, [Fl]; Sb, Bi; and Po.[123][n 18]
الفلزات الأقل نمطية
Habashi[125] groups the elements into eight major categories: [1] typical metals (alkali metals, alkaline earth metals, and aluminium); [2] lanthanides (Ce–Lu); [3] actinides (Th–Lr); [4] transition metals (Sc, Y, La, Ac, groups 4–10); [5] less typical metals (groups 11–12, Ga, In, Tl, Sn and Pb); [6] metalloids (B, Si, Ge, As, Se, Sb, Te, Bi and Po); [7] covalent nonmetals (H, C, N, O, P, S and the halogens); and [8] monatomic nonmetals (that is, the noble gases).
اللافلزات
The metametals are zinc, cadmium, mercury, indium, thallium, tin and lead. They are ductile elements but, compared to their metallic periodic table neighbours to the left, have lower melting points, relatively low electrical and thermal conductivities, and show distortions from close-packed forms.[126] Sometimes beryllium[127] and gallium[128] are included as metametals despite having low ductility.
Ordinary metals
Abrikosov[129] distinguishes between ordinary metals, and transition metals where the inner shells are not filled. The ordinary metals have lower melting points and cohesive energies than those of the transition metals.[130] Gray[131] identifies as ordinary metals: aluminium, gallium, indium, thallium, element 113, tin, lead, element 114, bismuth, element 115, and element 116. He adds that, 'in reality most of the metals that people think of as ordinary are in fact transition metals...'.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
P-block metals
The p-block metals are the metals in groups 13‒15 (or 16) of the periodic table. Usually, this includes aluminium, gallium, indium and thallium; tin and lead; and bismuth. Germanium, antimony and polonium are sometimes also included, although the first two are commonly recognised as metalloids. The p-block metals tend to have structures that display low coordination numbers and directional bonding. Pronounced covalency is found in their compounds; the majority of their oxides are amphoteric.[132]
Peculiar metals
Slater[133] divides the metals 'fairly definitely, though not perfectly sharply' into the ordinary metals and the peculiar metals, the latter of which verge on the nonmetals. The peculiar metals occur towards the ends of the rows of the periodic table and include 'approximately:' gallium, indium, and thallium; carbon, silicon '(both of which have some metallic properties, though we have previously treated them as nonmetals),' germanium and tin; arsenic, antimony, and bismuth; and selenium '(which is partly metallic)' and tellurium. The ordinary metals have centro-symmetrical crystalline structures[n 19] whereas the peculiar metals have structures involving directional bonding. More recently, Joshua observed that the peculiar metals have mixed metallic-covalent bonding.[135]
الفلزات الفقيرة
Farrell and Van Sicien[136] use the term poor metal, for simplicity, 'to denote one with a significant covalent, or directional character.' Hill and Holman[137] observe that, 'The term poor metals is not widely used, but it is a useful description for several metals including tin, lead and bismuth. These metals fall in a triangular block of the periodic table to the right of the transition metals. They are usually low in the activity (electrochemical) series and they have some resemblances to non-metals.' Reid et al.[138] write that 'poor metals' is, '[A]n older term for metallic elements in Groups 13‒15 of the periodic table that are softer and have lower melting points than the metals traditionally used for tools.'
فلزات ما بعد الانتقالية
The term post-transition metal is generally used to describe the category of metallic elements in periods 4–6 of the periodic table, to the right of the transition elements. As this description excludes aluminium, a period 3 metal,[n 20] the post-transition elements thereby form a subset of the other metals. The post-transition metals generally show reduced electropositivity, anionic species formation and a capacity to combine with electropositive metals to give Zintl phases. Compounds of the group 12 metals (zinc, cadmium and mercury) are markedly non-ionic in character, both in structure and properties. Simple cationic chemistry in the group 14 metals, tin and lead, is the exception rather than the norm.[139]
فلزات انتقالية
Historically, the transition metal series 'includes those elements of the Periodic Table which "bridge the gap" between the very electropositive alkali and allkaline earth metals and the electronegative non-metals of the groups: nitrogen-phosphorus, oxygen-sulfur, and the halogens.'[140] Cheronis, Parsons and Ronneberg[141] wrote that, 'The transition metals of low melting point form a block in the Periodic Table: those of Groups II b [zinc, cadmium, mercury], III b [aluminium, gallium, indium, thallium], and germanium, tin and lead in Group IV. These metals all have melting points below 425 °C.'[n 21]
الهامش
- ^ Physical properties: 'The lighter alkaline earths possess fairly high electrical and thermal conductivities and sufficient strength for structural use. The heavier elements are poor conductors and are too weak and reactive for structural use.'[3] Chemical: The lighter alkaline earths show covalent bonding tendencies (Be predominately; Mg considerably) whereas compounds of the heavier alkaline earths are predominately ionic in nature; the heavier alkaline earths have more stable hydrides and less stable carbides.[4]
- ^ Moh's hardness values are taken from Samsanov,[5] unless otherwise noted; bulk coordination number values are taken from Darken and Gurry,[6] unless otherwise noted.
- ^ Francium may have a comparably low bonding energy but its melting point of around 27°C is significantly higher than that of mercury, at −39°C.
- ^ Mercury also forms partially anionic oxomercurates, such as Li2HgO2 and CdHgO4, by heating mixtures of HgO with the relevant cation oxides, including under oxygen pressure (Müller-Buschbaum 1995; Deiseroth 2004, pp. 173, 177, 185–186).
- ^ The partially directional bonding in aluminium improves its shear strength but means that ultrahigh-purity aluminium cannot maintain work hardening at room temperature.[29]
- ^ Without the use of thermal insulation and detailed structural design attention,[33] aluminium's low melting point and high thermal conductivity mitigate against its use, for example, in military ship construction—should a ship burn, the low melting point results in structural collapse; the high thermal conductivity helps spread the fire.[34] Its use in the construction of cargo ships is limited as little or no economic advantage is gained over steel, once the cost and weight of fitting thermal insulation is taken into account.[35]
- ^ Aluminium can be attacked, for example, by alkaline detergents[40] (including those used in dishwashers);[41] by wet concrete,[42] and by highly acidic foods such as tomatoes, rhubarb or cabbage.[43] It is not attacked by nitric acid.[44]
- ^ See the list of metalloid lists for references
- ^ Aluminium wire is used in electrical transmisson lines for the distribution of power but, on account of its low breaking strength, is refinforced with a central core of galvanised steel wire.[47]
- ^ In the absence of protective measures, the relatively high electropositivity of aluminium renders it susceptible to galvanic corrosion when in physical or electrical contact with other metals such as copper or steel, especially when exposed to saline media, such as sea water or wind-blown sea spray.[50]
- ^ Charles, Crane and Furness write that, 'Most metals, except perhaps lead and tin, can be alloyed to give [yield] strengths that lie in the upper two-thirds of the low-strength range…'[72]
- ^ Which metal has the lowest electrical conductivity is debatable but bismuth is certainly in the lowest cohort; Hoffman[98] refers to bismuth as 'a poor metal, on the verge of being a semiconductor.'
- ^ Bagnall[107] writes that the fusion of polonium dioxide with a potassium chlorate/hydroxide mixture yields a bluish solid which, '...presumably contains some potassium polonate.'
- ^ Bagnall[110] noted that the rare-earth polonides have the greatest thermal stability of any polonium compound.
- ^ Greenwood and Earnshaw[112] refer to the B-subgroup metals as post-transition elements: 'Arsenic and antimony are classed as metalloids or semi-metals and bismuth is a typical B sub-group (post-transition-element) metal like tin and lead.'
- ^ Aluminium is identified by Parish, along with germanium, antimony and bismuth, as being a metal on the boundary line between metals and non-metals; he suggests that all these elements are 'probably better classed as metalloids.'[118]
- ^ Pauling,[120] in contrast, refers to the strong metals in Groups 1 and 2 (that form ionic compounds with 'the strong nonmetals in the upper right corner of the periodic table.').
- ^ Hawkes,[124] attempting to address the question of what is a heavy metal, commented that, 'Being a heavy metal has little to do with density, but rather concerns chemical properties'. He observed that, 'It may mean different things to different people, but as I have used, heard and interpreted the term over the last half-century, it refers to metals with insoluble sulfides and hydroxides, whose salts produce colored solutions in water, and whose complexes are usually colored.' He goes on to note that, 'The metals I have seen referred to as heavy metals comprise a block of all the metals in Groups 3 to 16 that are in periods 4 and greater. It may also be stated as the transition metals and post-transition metals.
- ^ On manganese, Slater says, '[It] is a very peculiar and anomalous exception to the general order of the elements. It is the only definite metal, far from the nonmetals in the table, which has a complicated structure.'[134]
- ^ Aluminium is sometimes referred to as a pre-transition metal, along with the group 1 alkali metals and group 2 alkaline earth metals.
- ^ In fact, both aluminium (660.32) and germanium (938.25) have melting points greater than 425°C.
Citations
- ^ Roher 2001, pp. 2‒3
- ^ Messler 2006, p. 347
- ^ Russell & Lee 2005, p. 165
- ^ Cotton et al. 1999, pp. 111–113; Greenwood & Earnshaw 2002, p. 111–113
- ^ Samsanov 1968
- ^ Darken & Gurry 1953, pp. 50–53
- ^ Schweitzer 2003, p. 603
- ^ Hutchinson 1964, p. 562
- ^ Greenwood & Earnshaw 1998, p. 1209; Gupta CK 2002, p. 590
- ^ أ ب ت ث ج Rayner-Canham & Overton 2006, p. 30
- ^ أ ب Kneip 1996, p. xxii
- ^ Russell & Lee 2005, p. 339
- ^ Sequeira 2013, p. 243
- ^ أ ب Russell & Lee 2005, p. 349
- ^ Borsari 2005, p. 608
- ^ Dirkse 1986, pp. 287–288, 296; Ivanov-Emin, Misel'son & Greksa 1960
- ^ Wanamaker & Pennington 1921, p. 56
- ^ Rayner-Canham 2006, p. 570; Chambers & Holliday 1975, p. 58; Wiberg, Holleman & Wiberg 2001, p. 247; Aylward & Findlay 2008, p. 4
- ^ Poole 2004, p. 821
- ^ Mittemeijer 2010, p. 138
- ^ Russell & Lee 2005, pp. 1–2; 354
- ^ Rayner-Canham 2006, p. 567
- ^ Moeller 1952, pp. 859, 866
- ^ Cooney & Hall 1966, p. 2179
- ^ Deiseroth 2008, pp. 179‒180; Sevov 1993
- ^ Russell & Lee 2005, p. 354
- ^ Gerard & King 1968, p. 16; Dwight 1999, p. 2
- ^ Russell & Lee 2005, pp. 1–2; 359
- ^ Ogata, Li & Yip 2002; Russell & Lee 2005, p. 360; Glaeser 1992, p. 224
- ^ Lyons 2004, p. 170
- ^ Cobb 2009, p. 323
- ^ Polemear 2006, p. 184
- ^ Holl 1989, p. 90
- ^ Ramroth 2006, p. 6; US Dept. of Transportation, Maritime Administration 1987, pp. 97, 358
- ^ Noble 1985, p. 21
- ^ Cooper 1968, p. 25; Henderson 2000, p. 5
- ^ Kauzlarich 2005, pp. 6009–10
- ^ Dennis & Such 1993, p. 391
- ^ Cramer & Covino 2006, p. 25
- ^ Hinton & Dobrota 1978, p. 37
- ^ Holman & Stone 2001, p. 141
- ^ Hurd 2005, p. 4-15
- ^ Vargel 2004, p. 580
- ^ Hill & Holman 2000, p. 276
- ^ Russell & Lee 2005, p. 360
- ^ Clegg & Dovaston 2003, p. 5/5
- ^ Liptrot 2001, p. 181
- ^ Kent 1993, pp. 13–14
- ^ Steele 1966, p. 60
- ^ Davis 1999, p. 75–7
- ^ أ ب Russell & Lee 2005, p. 387
- ^ Driess 2004, p. 151; Donohue 1982, p. 237
- ^ Walker, Enache & Newman 2013, p. 38
- ^ Atkins et al. 2006, p. 123
- ^ Corbett 1996, p. 161
- ^ Eranna 2012, p. 67
- ^ Chandler 1998, p. 59
- ^ أ ب Russell & Lee 2005, p. 389
- ^ أ ب Evans 1966, p. 129–130
- ^ Liang, King & White 1968, p. 288
- ^ Busev 1962, p. 33; Liang, King & White 1968, p. 287; Solov'eva et al. 1973, p. 43; Greenwood & Earnshaw 1998, p. 226; Leman & Barron 2005, p. 1522
- ^ Kneip 1996, p. xxii; Corbett 1996, pp. 153, 158
- ^ Russell & Lee 2005, p. 390
- ^ أ ب Wells 1985, p. 1279–80
- ^ Howe 1968a, p. 709; Taylor & Brothers 1993, p. 131; Lidin 1996, p. 410; Tóth & Győri 2005, pp. 4, 6–7
- ^ Chambers & Holliday 1975, p. 144
- ^ أ ب Bashilova & Khomutova 1984, p. 1546
- ^ Ropp 2012, p. 484
- ^ King & Schleyer 2004, p. 19
- ^ Corbett 1996, p. 153; King 2004, p. 199
- ^ أ ب Russell & Lee 2005, p. 405
- ^ Charles, Crane & Furness 1997, pp. 49, 57
- ^ Rayner-Canham 2006, pp. 306, 340
- ^ Wiberg, Holleman & Wiberg 2001, p. 247
- ^ Corbett 1996, p. 143; Cotton et al. 1999, pp. 99, 122; Kauzlarich 2005, p. 6009
- ^ Russell & Lee 2005, pp. 402, 405
- ^ Russell & Lee 2005, p. 402, 407
- ^ Alhassan & Goodwin 2005, p. 532
- ^ Schweitzer 2003, p. 695
- ^ Mackay & Mackay 1989, p. 86; Norman 1997, p. 36
- ^ Hutchinson 1959, p. 455; Wells 1984, p. 1188; Liu, Knowles & Chang 1995, p. 125; Bharara & Atwood 2005, pp. 2, 4
- ^ Durrant & Durrant 1970, p. 670; Lister 1998, p. A12; Cox 2004, p. 204
- ^ Patnaik 2003, p. 474
- ^ Corbett 1996, pp. 143, 147; Cotton et al. 1999, p. 122; Kauzlarich 2005, p. 6009
- ^ Russell & Lee 2005, pp. 411, 13
- ^ Russell & Lee 2005, p. 428
- ^ Eagleson 1994, p. 282
- ^ Russell & Lee 2005, p. 427
- ^ Sidgwick 1937, p. 181
- ^ Howe 1968, p. 62
- ^ Durrant & Durrant 1970, p. 790
- ^ Wiberg, Holleman & Wiberg 2001, p. 771; McQuarrie, Rock & Gallogly 2010, p. 111
- ^ Ropp 2012, p. 328
- ^ Miller, Lee & Choe 2002, p. 14; Aleandri & Bogdanović 2008, p. 326
- ^ Bobev & Sevov 2002
- ^ Xia & Bobev 2006
- ^ Bailar et al. 1984, p. 951
- ^ Hoffman 2004
- ^ Beamer & Maxwell 1946, pp. 1, 31
- ^ Russell & Lee 2005, p. 431
- ^ Halford 2006,p. 378
- ^ Legut, Friák & Šob 2010
- ^ Wiberg, Holleman & Wiberg 2001, pp. 594; Petrii 2012, p. 754
- ^ Bagnall 1966, p. 83
- ^ Bagnall 1966, pp. 42, 61; Wiberg, Holleman & Wiberg 2001, pp. 767–68
- ^ Schwietzer & Pesterfield pp. 241, 243
- ^ Bagnall 1962, p. 211
- ^ Wiberg, Holleman & Wiberg 2001, pp. 283, 595
- ^ Greenwood & Earnshaw 1998, p. 766
- ^ Bagnall 1966, p. 47
- ^ Zubieta & Zuckerman 2009, p. 260: 'The compounds AsSn and SbSn, which are classified as alloys of two B subgroup metals, exhibit superconducting properties with a transition temperature of about 4 K.'; Schwartz 2010, p. 32: 'The metals include the alkali and alkaline earths, beryllium, magnesium, copper, silver, gold and the transition metals. These metals exhibit those characteristics generally associated with the metallic state. The B subgroups comprise the remaining metallic elements. These elements exhibit complex structures and significant departures from typically metallic properties. Aluminum, although considered under the B subgroup metals, is somewhat anomolous as it exhibits many characteristics of a true metal.'
- ^ Greenwood & Earnshaw 1998, p. 548
- ^ Phillips & Williams 1965, pp. 4‒5; Steele 1966, p. 66
- ^ Phillips & Williams 1965, p. 33
- ^ Wiberg, Holleman & Wiberg 2001, pp. 1253, 1268
- ^ Steele 1966, p. 67
- ^ Parish 1977, pp. 201–202
- ^ Parish 1977, pp. 178
- ^ Rayner-Canham & Overton 2006, p. 29‒30
- ^ Pauling 1988, p. 173
- ^ Van Wert 1936, pp. 16, 18
- ^ Britton, Abbatiello & Robins 1972, p. 704
- ^ Sargent-Welch 2008
- ^ Hawkes 1997
- ^ Habashi 2010
- ^ Wiberg, Holleman & Wiberg 2001, p. 143
- ^ Klemm 1950
- ^ Miller GJ, Lee C & Choe W 2002, p. 22
- ^ Abrikosov 1988, p. 31
- ^ Cremer 1965, p. 514
- ^ Gray 2009, p. 9
- ^ Parish 1977, pp. 178, 189–190, 192–3
- ^ Slater 1939, p.&npsb;444‒445
- ^ Slater 1939, p. 448
- ^ Joshua 1991, p. 45
- ^ Farrell & Van Sicien 2007, p. 1442
- ^ Hill & Holman 2000, p. 40
- ^ Reid 2011, p. 143
- ^ Cox 2004, pp. 27, 112, 185, 196, 202–3
- ^ Young et al. 1969, p. 228
- ^ Cheronis, Parsons & Ronneberg 1942, p. 570
شاهد أيضا
المصادر
- ويكيبيديا الإنجليزية.









