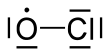أحادي أكسيد الكلور
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك المفضل
Chlorine monoxide | |||
| اسم أيوپاك النظامي
Chlorooxidanyl | |||
| أسماء أخرى
Chlorine(II) oxide
| |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| اختصارات | ClO• | ||
| ChEBI | |||
| ChemSpider | |||
| عناوين مواضيع طبية MeSH | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| الخصائص | |||
| الصيغة الجزيئية | ClO | ||
| كتلة مولية | 51.45 g mol-1 | ||
| الكيمياء الحرارية | |||
| الإنتالپية المعيارية للتشكل ΔfH |
101.8 kJ/mol[1] | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
أحادي أكسيد الكلور مركب كيميائي له الصيغة ClO . ويلعب دوراً هاماً في عملية ozone depletion. يتشكل في الستراتوسفير أثناء تخريب طبقة الأوزون بغاز الكلور
Cl + O3 → ClO + O2.
This reaction causes the depletion of the ozone layer.[1] The resulting ClO• radicals can further react:
- ClO• + O• → Cl• + O2
regenerating the chlorine radical. In this way, the overall reaction for the decomposition of ozone is catalyzed by chlorine, as ultimately chlorine remains unchanged. The overall reaction is:
- O• + O3 → 2 O2
There has been a significant impact of the use of CFCs on the upper stratosphere, although many countries have agreed to ban the use of CFCs. The nonreactive nature of CFCs allows them to pass into the stratosphere, where they undergo photo-dissociation to form Cl radicals. These then readily form chlorine monoxide, and this cycle can continue until two radicals react to form dichlorine monoxide, terminating the radical reaction. Because the concentration of CFCs in atmosphere is very low, the probability of a terminating reaction is exceedingly low, meaning each radical can decompose many thousands of molecules of ozone.
Even though the use of CFCs has been banned in many countries, CFCs can stay in the atmosphere for 50 to 500 years. This causes many chlorine radicals to be produced and hence a significant amount of ozone molecules are decomposed before the chlorine radicals are able to react with chlorine monoxide to form dichlorine monoxide.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
المصادر
- ^ أ ب Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 462. ISBN 0-12-352651-5.