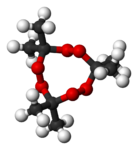
پروكسيد الأسيتون
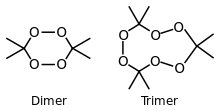 [Cyclic dimer and trimer examples] | |||
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاكs
3,3-Dimethyl-1,2-dioxacyclopropane
(monomer) 3,3,6,6-Tetramethyl-1,2,4,5-tetraoxane (dimer) 3,3,6,6,9,9-Hexamethyl-1,2,4, 5,7,8-hexaoxacyclononane (trimer) 3,3,6,6,9,9,12,12-Octamethyl-1,2,4, 5,7,8,10,11-octaoxacyclododecane (tetramer) | |||
| أسماء أخرى
Triacetone Triperoxide
Peroxyacetone Mother of Satan | |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
| E number | E929 (glazing agents, ...) | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| الخصائص | |||
| الصيغة الجزيئية | C6H12O4 (dimer) C9H18O6 (trimer) C12H24O8 (tetramer) | ||
| كتلة مولية | 148.157 g/mol (dimer) 222.24 g/mol (trimer) | ||
| المظهر | White crystalline solid | ||
| نقطة الانصهار | |||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | insoluble | ||
| المخاطر | |||
| خطر رئيسي | Explosive | ||
| بيانات متفجر | |||
| حساسية الصدم | High / moderate when wet | ||
| حساسية الاحتكاك | High / moderate when wet | ||
| RE factor | 0.83 | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
پروكسيد الأسيتون Acetone peroxide هو تعبير كيميائي يشير إلى عائلة صغيرة من الپروكسيدات العضوية المتفجرة that are tetrameric, trimeric, dimeric, and monomeric peroxides of acetone, which can take cyclic or open chain forms. One frequently studied is the trimer, triacetone triperoxide (TATP; synonyms, tri-cyclic acetone peroxide, TCAP; peroxyacetone). Some forms of these peroxides take the form of a crystalline powders. Most can explode if subjected to heat, friction, or shock, and are considered high explosives. As a non-nitrogenous explosive, TATP has historically been more difficult to detect, and it has been implicated as the primary high explosive used in terrorist attacks in Europe in 2016 and earlier.
التاريخ
Acetone peroxide was discovered in 1895 by Richard Wolffenstein.[1][2] He was the first researcher to receive a patent for using the peroxide as an explosive compound.
In 1900 Bayer and Villiger described the first synthesis of the dimer and described use of acids for the synthesis of both peroxides.[3] Work on this methodology, and on the various products obtained, was further investigated in the mid-20th century by Milas and Golubović.خطأ استشهاد: إغلاق </ref> مفقود لوسم <ref> Because of its high susceptibility to accidental detonation (and resulting "workplace accidents" in bomb-making shops), TATP has been referred to as the "Mother of Satan."[4] It is used by terrorists for its ability to evade detection aimed at nitrogenous explosives, and due to its low cost and the ease with which its precursors can be obtained.[4] It has been described in popular media as easily prepared from readily available retail ingredients, such as hair bleach and nail polish remover.[5]
As such, TATP has been used in bomb and suicide attacks and in improvised explosive devices, including in Israel,[بحاجة لمصدر] and in the London bombings on 7 July 2005, where four suicide bombers killed 52 people and injured more than 700.[6][7] It was one of the explosives used by the "shoe bomber" Richard Reid[7] and was used by the suicide bombers in the November 2015 Paris attacks[5] and 2016 Brussels bombings.[8]
الهامش
- ^ Wolffenstein, R (1895). "Über die Einwirkung von Wasserstoffsuperoxyd auf Aceton und Mesityloxyd (On the effect of hydrogen peroxide on acetone and mesityl oxide)". Berichte der Deutschen chemischen Gesellschaft. 28 (2): 2265–2269. doi:10.1002/cber.189502802208.
- ^ Wolffenstein, Richard (1895) Deutsches Reich Patent 84,953.
- ^ Baeyer, Adolf; Villiger, Victor (1900). "Über die Einwirkung des Caro'schen Reagens auf Ketone". Berichte der deutschen chemischen Gesellschaft. 33 (1): 858–864. doi:10.1002/cber.190003301153.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help);[بحاجة لمصدر غير رئيسي] See also Baeyer, Adolf; Villiger, Victor (1900). "Über die Nomenclatur der Superoxyde und die Superoxyde der Aldehyde". Berichte der deutschen chemischen Gesellschaft. 33 (2): 2479–2487. doi:10.1002/cber.190003302185.{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)[بحاجة لمصدر غير رئيسي] - ^ أ ب "TATP: Countering the Mother of Satan". The Future of Things. 2006-11-06. Retrieved 24 September 2009.
The tremendous devastative force of TATP, together with the relative ease of making it, as well as the difficulty in detecting it, made TATP one of the weapons of choice for terrorists
{{cite web}}: Cite uses deprecated parameter|authors=(help) - ^ أ ب "A View of ISIS's Evolution in New Details of Paris Attacks". The New York Times. 2016-03-19.
- ^ Naughton, Philippe (2005-07-15). "TATP is suicide bombers' weapon of choice". The Times (UK).
- ^ أ ب Vince, Gaia (15 July 2005). "Explosives linked to London bombings identified". New Scientist.
- ^ (بالفرنسية) "La mère de Satan" ou TATP, l'explosif préféré de l'EI
وصلات خارجية
- CS1 errors: unsupported parameter
- All pages needing factual verification
- Wikipedia articles needing factual verification from March 2016
- CS1 errors: deprecated parameters
- E number from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- مقالات ذات عبارات بحاجة لمصادر
- كيماويات متفجرة
- كتالات
- پروكسيدات عضوية
- حلقات غير متجانسة أكسجينية
- بادئات جذرية