ليسينوپريل
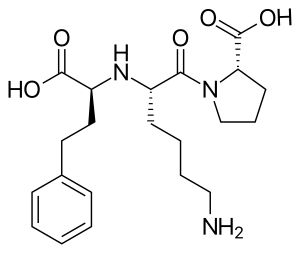 | |
 التركيب الكيميائي لليسينوپريل | |
| البيانات السريرية | |
|---|---|
| النُطق | /laɪˈsɪnəprɪl/, ly-SIN-ə-pril |
| الأسماء التجارية | Prinivil,[1] Zestril,[2] Qbrelis,[3] others[4] |
| أسماء أخرى | (2S)-1-[(2S)-6-amino-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic acid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692051 |
| License data |
|
| فئة السلامة أثناء الحمل | |
| مسارات الدواء | عن طريق الفم |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | حوالي 25%، لكن يختلف على نطاق واسع بين الأفراد (6 إلى 60%) |
| ارتباط الپروتين | 0 |
| الأيض | لا |
| Elimination half-life | 12 ساعة |
| الإخراج | يطرح دون تغير في البول |
| المعرفات | |
| |
| رقم CAS |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.332 |
| Chemical and physical data | |
| التركيب | C21H31N3O5 |
| الكتلة المولية | 405٫50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
الليسينوپريل (إنگليزية: Lisinopril)، هو دواء من فئة مثبطات الإنزيم المحول للأنجيوتنسين المستخدم لعلاج فرط ضغط الدم، قصور القلب واحتشاء عضل القلب واعتلال الكلى السكري.[6] لعلاج فرط ضغط الدم، عادة ما يكون علاج من الخط الأول. كما يستخدم للوقاية من مشكلات الكلى لدى الأشخاص المصابين بمرض السكري.[6] يتم تناوله عن طريق الفم.[6] كي تظهر التأثيرات الكاملة للدواء يجب استخدامه أربع أسابيع.[6]
تتضمن الآثار الجانبية الصداع، الدوخة، الشعور بالتعب، السعال، الغثيان، والطفح الجلدي. [6] قد تشمل الآثار الجانبية الخطيرة انخفاض ضغط الدم، مشاكل في الكبد، ارتفاع پوتاسيوم الدم، والوذمة الوعائية.[6] Use is not recommended during the entire duration of pregnancy as it may harm the baby.[6] Lisinopril works by inhibiting the renin–angiotensin–aldosterone system.[6]
Lisinopril was patented in 1978, and approved for medical use in the United States in 1987.[6][8] It is available as a generic medication.[6] In 2018, it was the third most commonly prescribed medication in the United States, with more than 97 million prescriptions.[9][10] In July 2016, an oral solution formulation of lisinopril was approved for use in the United States.[6][11]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الاستخدامات الطبية
Lisinopril is typically used for the treatment of high blood pressure, congestive heart failure, acute myocardial infarction (heart attack), and diabetic nephropathy.[6][1]
A review concluded that lisinopril was effective for treatment of proteinuric kidney disease, including diabetic proteinuria.[12]
In people of sub-Saharan African descent, calcium-channel blockers or thiazide diuretics are more effective than lisinopril for lowering blood pressure,[6] although there is not convincing evidence that these drugs differ in their effect on morbidity or mortality in such persons.[13]
مشكلات الكلى
The dose must be adjusted in those with poor kidney function. Dose adjustments may be required when creatinine clearance is less than or equal to 30mL/min.[1] Since lisinopril is removed by dialysis, dosing changes must also be considered for people on dialysis.[1]
الحمل والرضاعة الطبيعية
Animal and human data have revealed evidence of lethal harm to the embryo and teratogenicity associated with ACE inhibitors. No controlled data in human pregnancy are available. Birth defects have been associated with use of lisinopril in any trimester. However, there have been reports of death and increased toxicity to the fetus and newly born child with the use of lisinopril in the second and third trimesters. The label states, "When pregnancy is detected, discontinue Zestril as soon as possible." The manufacturer recommends mothers should not breastfeed while taking this medication because of the current lack of safety data.[1]
موانع الاستخدام
Lisinopril is contraindicated in people who have a history of angioedema (hereditary or idiopathic) or who have diabetes and are taking aliskiren.[1]
الآثار الجانبية
The incidence of adverse effects varies according to which disease state the patient is being treated for.[1]
People taking lisinopril for the treatment of hypertension may experience the following side effects:[1][المصدر لا يؤكد ذلك]
- Headache (3.8%)
- Dizziness (3.5%)
- Cough (2.5%) Persons with the ACE I/D genetic polymorphism may be at higher risk for ACE inhibitor-associated cough.[14]
- Difficulty swallowing or breathing (signs of angioedema), allergic reaction (anaphylaxis)
- Hyperkalemia (2.2% in adult clinical trials)
- Fatigue (1% or more)
- Diarrhea (1% or more)
- Some severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens–Johnson syndrome; causal relationship has not been established.
People taking lisinopril for the treatment of myocardial infarction may experience the following side effects:[1][المصدر لا يؤكد ذلك]
- Hypotension (5.3%)
- Kidney dysfunction (1.3%)
People taking lisinopril for the treatment of heart failure may experience the following side effects:[1][المصدر لا يؤكد ذلك]
- Hypotension (3.8%)
- Dizziness (12% at low dose – 19% at high dose)
- Chest pain (2.1%)
- Fainting (5–7%)
- Hyperkalemia (3.5% at low dose – 6.4% at high dose)
- Difficulty swallowing or breathing (signs of angioedema), allergic reaction (anaphylaxis)
- Fatigue (1% or more)
- Diarrhea (1% or more)
- Some severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens–Johnson syndrome; a causal relationship has not been established.
الجرعة الزائدة
In one reported overdose, the half-life of lisinopril was prolonged to 14.9 hours.[15] The case report of the event estimates that the individual consumed between 420 and 500 mg of lisinopril and survived.[16] In cases of overdosage, it can be removed from circulation by dialysis.[1]
التفاعلات الدوائية
- Dental care
ACE-inhibitors like lisinopril are considered to be generally safe for people undergoing routine dental care, though the use of lisinopril prior to dental surgery is more controversial, with some dentists recommending discontinuation the morning of the procedure.[17] People may present to dental care suspicious of an infected tooth, but the swelling around the mouth may be due to lisinopril-induced angioedema, prompting emergency, and medical referral.[17]
علم الصيدلة
Lisinopril is the lysine-analog of enalapril. Unlike other ACE inhibitors, it is not a prodrug, is not metabolized by the liver, and is excreted unchanged in the urine.[1]
آلية العمل
Lisinopril is an ACE inhibitor, meaning it blocks the actions of angiotensin-converting enzyme (ACE) in the renin–angiotensin–aldosterone system (RAAS), preventing angiotensin I from being converted to angiotensin II. Angiotensin II is a potent direct vasoconstrictor and a stimulator of aldosterone release. Reduction in the amount of angiotensin II results in relaxation of the arterioles. Reduction in the amount of angiotensin II also reduces the release of aldosterone from the adrenal cortex, which allows the kidney to excrete sodium along with water into the urine, and increases retention of potassium ions.[18] Specifically, this process occurs in the peritubular capillaries of the kidneys in response to a change in Starling forces.[19] The inhibition of the RAAS system causes an overall decrease in blood pressure.[18]
Pharmacokinetics
الامتصاص
Lisinopril has a poor bioavailability of ~25% (reduced to 16% in people with New York Heart Association Functional Classification (NYHA) Class II–IV heart failure).[1][18] Its time to peak concentration is 7 hours.[1][18] The peak effect of lisinopril is about 4 to 8 hours after administration.[20] Food does not affect its absorption.[20]
الانتشار
Lisinopril does not bind to proteins in the blood.[1][18] It does not distribute as well in people with NYHA Class II–IV heart failure.[1][18]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الأيض
Lisinopril is the only water-soluble member of the ACE inhibitor class, with no metabolism by the liver.[20]
Elimination
Lisinopril leaves the body completely unchanged in the urine.[1][18] The half-life of lisinopril is 12 hours, and is increased in people with kidney problems.[1][18] While the plasma half-life of lisinopril has been estimated between 12–13 hours, the elimination half-life is much longer, at around 30 hours.[20] The full duration of action is between 24 and 30 hours.[20]
الكيمياء
Pure lisinopril powder is white to off white in color.[1] Lisinopril is soluble in water (approximately 13 mg/L at room temperature),[21] less soluble in methanol, and virtually insoluble in ethanol.[1]
التاريخ
Captopril, the first ACE inhibitor, is a functional and structural analog of a peptide derived from the venom of the jararaca, a Brazilian pit viper (Bothrops jararaca).[22] Enalapril is a derivative, designed by scientists at Merck to overcome the rash and bad taste caused by captopril.[23][24] Enalapril is actually a prodrug; the active metabolite is enalaprilat.[25]
Lisinopril is a synthetic peptide derivative of captopril.[21] Scientists at Merck created lisinopril by systematically altering each structural unit of enalaprilat, substituting various amino acids. Adding lysine at one end of the drug turned out to have strong activity and had adequate bioavailability when given orally; analogs of that compound resulted in lisinopril, which takes its name from the discovery with lysine. Merck conducted clinical trials, and the drug was approved for hypertension in 1987 and congestive heart failure in 1993.[25]
The discovery posed a problem, since sales of enalapril were strong for Merck, and the company did not want to diminish those sales. Merck ended up entering into an agreement with Zeneca under which Zeneca received the right to co-market lisinopril, and Merck received the exclusive rights to an earlier stage aldose reductase inhibitor drug candidate, a potential treatment for diabetes. Zeneca's marketing and brand name, "Zestril", turned out to be stronger than Merck's effort.[26] The drug became a blockbuster for Astrazeneca (formed in 1998), with annual sales in 1999 of $1.2B.[27]
The US patents expired in 2002.[27] Since then, lisinopril has been available under many brand names worldwide; some formulations include the diuretic hydrochlorothiazide.[4]
المصادر
- ^ أ ب ت ث ج ح خ د ذ ر ز س ش ص ض ط ظ ع غ ف ق "Prinivil- lisinopril tablet". DailyMed. 4 November 2019. Retrieved 27 February 2020.
- ^ أ ب "Zestril- lisinopril tablet". DailyMed. 31 October 2019. Retrieved 5 August 2020.
- ^ أ ب "Qbrelis- lisinopril solution". DailyMed. 31 March 2020. Retrieved 5 August 2020.
- ^ أ ب "Lisinopril". Drugs.com. Retrieved 23 December 2018.
- ^ أ ب ت "Lisinopril Use During Pregnancy". Drugs.com. 22 October 2019. Retrieved 27 February 2020.
- ^ أ ب ت ث ج ح خ د ذ ر ز س ش ص "Lisinopril Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 23 December 2018.
- ^ "Summary of Product Characteristics (SmPC) – (emc)". Lisinopril 10mg Tablet. 13 November 2019. Retrieved 27 February 2020.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 467. ISBN 978-3527607495.
- ^ "The Top 300 of 2021". ClinCalc. Retrieved 18 February 2021.
- ^ "Lisinopril – Drug Usage Statistics". ClinCalc. Retrieved 18 February 2021.
- ^ "Drug Approval Package: Qbrelis (lisinopril)". U.S. Food and Drug Administration (FDA). 29 July 2016. Retrieved 5 August 2020.
- ^ Sadat-Ebrahimi SR, Parnianfard N, Vahed N, Babaei H, Ghojazadeh M, Tang S, Azarpazhooh A (July 2018). "An evidence-based systematic review of the off-label uses of lisinopril". Br J Clin Pharmacol. 84 (11): 2502–21. doi:10.1111/bcp.13705. PMC 6177695. PMID 29971804.
- ^ Seeley A, Prynn J, Perera R, Street R, Davis D, Etyang AO (March 2020). "Pharmacotherapy for hypertension in Sub-Saharan Africa: a systematic review and network meta-analysis". BMC Med. 18 (1): 75. doi:10.1186/s12916-020-01530-z. PMC 7099775. PMID 32216794.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mu G, Xiang Q, Zhou S, Xie Q, Liu Z, Zhang Z, Cui Y (January 2019). "Association between genetic polymorphisms and angiotensin-converting enzyme inhibitor-induced cough: a systematic review and meta-analysis". Pharmacogenomics. 20 (3): 189–212. doi:10.2217/pgs-2018-0157. PMID 30672376.
- ^ "HSDB: LISINOPRIL". toxnet.nlm.nih.gov. U.S. National Library of Medicine. Retrieved 6 June 2018.
- ^ Dawson, AH; Harvey, D; Smith, AJ; Taylor, M; Whyte, IM; Johnson, CI; Cubela, RB; Roberts, MJ (24 February 1990). "Lisinopril overdose". Lancet. 335 (8687): 487–88. doi:10.1016/0140-6736(90)90731-j. PMID 1968218. S2CID 8480643.
- ^ أ ب Weinstock, Robert; Johnson, Michael (2016). "Review of Top 10 Prescribed Drugs and Their Interaction with Dental Treatment". Dental Clinics of North America. 60 (2): 421–34. doi:10.1016/j.cden.2015.11.005. PMID 27040293.
- ^ أ ب ت ث ج ح خ د Katzung, Bertram (2012). Basic and Clinical Pharmacology. New York: McGraw Hill. pp. 175, 184–85. ISBN 978-0-07-176401-8.
- ^ Reddi, Alluru (2018). "Disorders of ECF Volume: Nephrotic Syndrome". Fluid, Electrolyte and Acid-Base Disorders. Springer. ISBN 978-3-319-60167-0.
- ^ أ ب ت ث ج Khan, M. Gabriel (2015). "Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers". Cardiac Drug Therapy. New York: Springer. ISBN 978-1-61779-962-4.
- ^ أ ب "Lisinopril". PubChem. Retrieved 6 June 2018.
- ^ Patlak M (March 2004). "From viper's venom to drug design: treating hypertension". FASEB J. 18 (3): 421. doi:10.1096/fj.03-1398bkt. PMID 15003987. S2CID 1045315.
- ^ Jenny Bryan for The Pharmaceutical Journal, 17 Apr 2009 "From snake venom to ACE inhibitor – the discovery and rise of captopril"
- ^ Jie Jack Li, "History of Drug Discovery". Chapter 1 in Drug Discovery: Practices, Processes, and Perspectives. Eds. Jie Jack Li, E. J. Corey. John Wiley & Sons, 2013 ISBN 978-1118354469
- ^ أ ب Menard J and Patchett A. "Angiotensin-Converting Enzyme Inhibitors". pp. 14–76 in Drug Discovery and Design. Volume 56 of Advances in Protein Chemistry. Eds. Richards FM, Eisenberg DS, and Kim PS. Series Ed. Scolnick EM. Academic Press, 2001. ISBN 978-0080493381. pp. 30–33
- ^ David R. Glover. Vie D'or: Memoirs of a Pharmaceutical Physician. Troubador Publishing Ltd, 2016. ISBN 978-1785894947. Merck Sharp and Dohme: lisinopril section
- ^ أ ب Express Scripts. Patent expirations
قراءات إضافية
- Fogari R, Zoppi A, Corradi L, Lazzari P, Mugellini A, Lusardi P (November 1998). "Comparative effects of lisinopril and losartan on insulin sensitivity in the treatment of non diabetic hypertensive patients". Br J Clin Pharmacol. 46 (5): 467–71. doi:10.1046/j.1365-2125.1998.00811.x. PMC 1873694. PMID 9833600.
- Bussien JP, Waeber B, Nussberger J, Gomez HJ, Brunner HR (1985). "Once-daily lisinopril in hypertensive patients: Effect on blood pressure and the renin–angiotensin system". Curr Therap Res. 37: 342–51.
وصلات خارجية
- "Lisinopril". Drug Information Portal. U.S. National Library of Medicine.
- CS1 maint: unflagged free DOI
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Multiple chemicals in Infobox drug
- Chemicals using indexlabels
- Chemical articles with multiple CAS registry numbers
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles containing إنگليزية-language text
- Pages using Lang-xx templates
- مقالات ذات عبارات بحاجة لمصادر
- ACE inhibitors
- Amino acid derivatives
- AstraZeneca brands
- Carboxamides
- Carboxylic acids
- Embryotoxicants
- Enantiopure drugs
- Merck & Co. brands
- پيروليدينات
- Teratogens
- Wikipedia medicine articles ready to translate