ثالث كبريتيد الزرنيخ

| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك المفضل
Arsenic trisulfide | |
أسماء أخرى
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.744 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | As2S3 |
| كتلة مولية | 246.04 g mol-1 |
| المظهر | yellow or orange crystals |
| الكثافة | 3.43 g/cm3 |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | insoluble |
| قابلية الذوبان | soluble in ammonia |
| القابلية المغناطيسية | −70.0·10−6 cm3/mol |
| البنية | |
| البنية البلورية | monoclinic |
| الزمرة الفراغية | P21/n (No. 11) |
| ثابت العقد | a = 1147.5(5) pm, b = 957.7(4) pm, c = 425.6(2) pm |
| ثابت العقد | α = 90°, β = 90.68(8)°, γ = 90° |
| pyramidal (As) | |
| المخاطر | |
| ن.م.ع. مخطط تصويري |  
|
| ن.م.ع. كلمة الاشارة | DANGER |
| H300, H331, H400, H411 | |
| NFPA 704 (معيـَّن النار) | |
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |
PEL (المسموح)
|
[1910.1018] TWA 0.010 mg/m3[1] |
REL (الموصى به)
|
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (خطر عاجل)
|
Ca [5 mg/m3 (as As)][1] |
| مركبات ذا علاقة | |
مركـّبات ذات علاقة
|
|
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
ثلاثي كبريتيد الزرنيخ مركب كيميائي له الصيغة As2S3 ، ويكون على شكل بلورات صفراء برتقالية. It is a dark yellow solid that is insoluble in water. It also occurs as the mineral orpiment (Latin: auripigmentum), which has been used as a pigment called King's yellow. It is produced in the analysis of arsenic compounds. It is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties.قالب:Cln
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
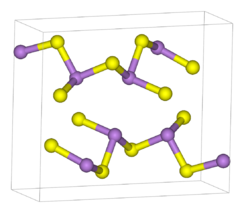
Structure
As
2S
3 occurs both in crystalline and amorphous forms. Both forms feature polymeric structures consisting of trigonal pyramidal As(III) centres linked by sulfide centres. The sulfide centres are two-fold coordinated to two arsenic atoms. In the crystalline form, the compound adopts a ruffled sheet structure.[4] The bonding between the sheets consists of van der Waals forces. The crystalline form is usually found in geological samples. Amorphous As
2S
3 does not possess a layered structure but is more highly cross-linked. Like other glasses, there is no medium or long-range order, but the first co-ordination sphere is well defined. As
2S
3 is a good glass former and exhibits a wide glass-forming region in its phase diagram.
Properties
It is a semiconductor, with a direct band gap of 2.7 eV.[5] The wide band gap makes it transparent to infrared light between 620 nm and 11 μm.
Synthesis
From the elements
Amorphous As
2S
3 is obtained via the fusion of the elements at 390 °C. Rapid cooling of the reaction melt gives a glass. The reaction can be represented with the chemical equation:
- 2 As + 3 S → As
2S
3
Aqueous precipitation
As
2S
3 forms when aqueous solutions containing As(III) are treated with H
2S. Arsenic was in the past analyzed and assayed by this reaction, which results in the precipitation of As
2S
3, which is then weighed. As
2S
3 can even be precipitated in 6 M HCl. As
2S
3 is so insoluble that it is not toxic.
Reactions
Upon heating in a vacuum, polymeric As
2S
3 "cracks" to give a mixture of molecular species, including molecular As
4S
6.[6][7] As
4S
6 adopts the adamantane geometry, like that observed for P
4O
6 and As
4O
6. When a film of this material is exposed to an external energy source such as thermal energy (via thermal annealing [8]), electromagnetic radiation (i.e. UV lamps, lasers,[9] electron beams)[10]), As4S6 polymerizes:
- 2 (As
2S
3)
n ⇌ n As
4S
6
As
2S
3 characteristically dissolves upon treatment with aqueous solutions containing sulfide ions.قالب:Cln The dissolved arsenic species is the pyramidal trithioarsenite anion AsS3−
3:
- As
2S
3 + 6 NaSH → 2 AsS3−
3 + 3 H
2Sقالب:Cln
As
2S
3 is the anhydride of the hypothetical trithioarsenous acid, As(SH)
3. Upon treatment with polysulfide ions, As
2S
3 dissolves to give a variety of species containing both S–S and As–S bonds. One derivative is S
7As−S−
, an eight-membered ring that contains 7 S atoms and 1 As atom, and an exocyclic sulfido center attached to the As atom. As
2S
3 also dissolves in strongly alkaline solutions to give a mixture of AsS3−
3 and AsO3−
3.[11]
"Roasting" As
2S
3 in air gives volatile, toxic derivatives, this conversion being one of the hazards associated with the refining of heavy metal ores:
- 2 As
2S
3 + 9 O
2 → As
4O
6 + 6 SO
2
Contemporary uses
As an inorganic photoresist
Due to its high refractive index of 2.45 and its large Knoop hardness compared to organic photoresists, As
2S
3 has been investigated for the fabrication of photonic crystals with a full-photonic band-gap. Advances in laser patterning techniques such as three-dimensional direct laser writing (3-D DLW) and chemical wet-etching chemistry, has allowed this material to be used as a photoresist to fabricate 3-D nanostructures.[12][13]
As
2S
3 has been investigated for use as a high resolution photoresist material since the early 1970s,[14][15] using aqueous etchants. Although these aqueous etchants allowed for low-aspect ratio 2-D structures to be fabricated, they do not allow for the etching of high aspect ratio structures with 3-D periodicity. Certain organic reagents, used in organic solvents, permit the high-etch selectivity required to produce high-aspect ratio structures with 3-D periodicity.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Medical applications
As
2S
3 and As
4S
4 have been investigated as treatments for acute promyelocytic leukemia (APL).
For IR-transmitting glasses
Arsenic trisulfide manufactured into amorphous form is used as a chalcogenide glass for infrared optics. It is transparent for light between wavelengths of 620 nm and 11 μm. The arsenic trisulfide glass is more resistant to oxidation than crystalline arsenic trisulfide, which minimizes toxicity concerns.[16] It can be also used as an acousto-optic material.
Arsenic trisulfide was used for the distinctive eight-sided conical nose over the infra-red seeker of the de Havilland Firestreak missile.
Role in ancient artistry
The ancient Egyptians reportedly used orpiment, natural or synthetic, as a pigment in artistry and cosmetics.
Miscellaneous
Arsenic trisulfide is also used as a tanning agent. It was formerly used with indigo dye for the production of pencil blue, which allowed dark blue hues to be added to fabric via pencil or brush.
Precipitation of arsenic trisulfide is used as an analytical test for presence of dissimilatory arsenic-reducing bacteria (DARB).[17]
Safety
As
2S
3 is so insoluble that its toxicity is low. Aged samples can contain substantial amounts of arsenic oxides, which are soluble and therefore highly toxic.
Natural occurrence
Orpiment is found in volcanic environments, often together with other arsenic sulfides, mainly realgar. It is sometimes found in low-temperature hydrothermal veins, together with some other sulfide and sulfosalt minerals.
References
- ^ أ ب ت NIOSH Pocket Guide to Chemical Hazards 0038
- ^ قالب:CLP Regulation
- ^ "Arsenic, inorganic compounds (as As)", 29 C.F.R. § 1910.1018, 58 FR 35310, June 30, 1993, as amended. قالب:PGCH-ref.
- ^ Wells, A.F. (1984). Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Arsenic sulfide (As2S3)
- ^ Martin, T.P. (1983). "Arsenic sulfide clusters". Solid State Communications. Elsevier BV. 47 (2): 111–114. Bibcode:1983SSCom..47..111M. doi:10.1016/0038-1098(83)90620-8. ISSN 0038-1098.
- ^ Hammam, M.; Santiago, J.J. (1986). "Evidence for As4S6 molecule as a structural model for amorphous arsenic sulfide from mass spectrometric analysis". Solid State Communications. Elsevier BV. 59 (11): 725–727. Bibcode:1986SSCom..59..725H. doi:10.1016/0038-1098(86)90705-2. ISSN 0038-1098.
- ^ Street, R. A.; Nemanich, R. J.; Connell, G. A. N. (1978-12-15). "Thermally induced effects in evaporated chalcogenide films. II. Optical absorption". Physical Review B. American Physical Society (APS). 18 (12): 6915–6919. Bibcode:1978PhRvB..18.6915S. doi:10.1103/physrevb.18.6915. ISSN 0163-1829.
- ^ Zoubir, Arnaud; Richardson, Martin; Rivero, Clara; Schulte, Alfons; Lopez, Cedric; et al. (2004-04-01). "Direct femtosecond laser writing of waveguides in As2S3 thin films". Optics Letters. The Optical Society. 29 (7): 748–50. Bibcode:2004OptL...29..748Z. doi:10.1364/ol.29.000748. ISSN 0146-9592. PMID 15072379.
- ^ Nordman, Olli; Nordman, Nina; Peyghambarian, Nasser (1998). "Electron beam induced changes in the refractive index and film thickness of amorphous AsxS100−x and AsxSe100−x films". Journal of Applied Physics. AIP Publishing. 84 (11): 6055–6058. doi:10.1063/1.368915. ISSN 0021-8979.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Wong, S.; Deubel, M.; Pérez-Willard, F.; John, S.; Ozin, G. A.; Wegener, M.; von Freymann, G. (2006-02-03). "Direct Laser Writing of Three- Dimensional Photonic Crystals with a Complete Photonic Bandgap in Chalcogenide Glasses". Advanced Materials. Wiley. 18 (3): 265–269. Bibcode:2006AdM....18..265W. doi:10.1002/adma.200501973. ISSN 0935-9648. S2CID 53527218.
- ^ Wong, Sean H.; Thiel, Michael; Brodersen, Peter; Fenske, Dieter; Ozin, Geoffrey A.; Wegener, Martin; von Freymann, Georg (2007). "Highly Selective Wet Etch for High-Resolution Three-Dimensional Nanostructures in Arsenic Sulfide All-Inorganic Photoresist". Chemistry of Materials. American Chemical Society (ACS). 19 (17): 4213–4221. doi:10.1021/cm070756y. ISSN 0897-4756.
- ^ Stoycheva, Rumiana; Simidchieva, Penka; Buroff, Atanas (1987). "Temperature dependence of the photodissociation of a-As2S3". Journal of Non-Crystalline Solids. Elsevier BV. 90 (1–3): 541–544. doi:10.1016/s0022-3093(87)80482-9. ISSN 0022-3093.
- ^ Zenkin, S. A.; Mamedov, S. B.; Mikhailov, M. D.; Turkina, E. Yu.; Yusupov, I. Yu. Glass Phys. Chem. 1997, 5, pp 393-399.
- ^ Material Safety Data Sheet Archived أكتوبر 7, 2007 at the Wayback Machine
- ^ Linping Kuai, Arjun A. Nair, and Martin F. Polz "Rapid and Simple Method for the Most-Probable-Number Estimation of Arsenic-Reducing Bacteria" Appl Environ Microbiol. 2001, vol. 67, 3168–3173. DOI:10.1128/AEM.67.7.3168-3173.2001.
المصادر
Further reading
- قالب:IARC arsenic.
- "Arsenic Compounds, Inorganic", Report on Carcinogens, Eleventh Edition (PDF), U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2005.
External links
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- Articles with hatnote templates targeting a nonexistent page
- Arsenic(III) compounds
- Sesquisulfides
- Optical materials
- Non-oxide glasses
- كبريتيدات
- مركبات الزرنيخ
- مواد بصرية
- زجاج غير أكسيدي
