تليوريد الزئبق
| |
| الأسماء | |
|---|---|
| اسم أيوپاك النظامي
Mercury telluride | |
| أسماء أخرى
Mercuric telluride, mercury(II) telluride
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.905 |
| رقم EC |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | HgTe |
| كتلة مولية | 328.19 g/mol |
| المظهر | near black cubic crystals |
| الكثافة | 8.1 g/cm3 |
| البنية | |
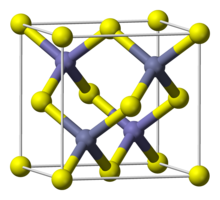
| البنية البلورية | Sphalerite, cF8 |
| الزمرة الفراغية | F43m, No. 216 |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride.
HgTe occurs in nature as the mineral form coloradoite.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الخصائص
All properties are at standard temperature and pressure unless stated otherwise. The lattice parameter is about 0.646 nm in the cubic crystalline form. The bulk modulus is about 42.1 GPa. The thermal expansion coefficient is about 5.2×10−6/K. Static dielectric constant 20.8, dynamic dielectric constant 15.1. Thermal conductivity is low at 2.7 W·m2/(m·K). HgTe bonds are weak leading to low hardness values. Hardness 2.7×107 kg/m2.[1][2][3]
Doping
N-type doping can be achieved with elements such as boron, aluminium, gallium, or indium. Iodine and iron will also dope n-type. HgTe is naturally p-type due to mercury vacancies. P-type doping is also achieved by introducing zinc, copper, silver, or gold.[1][2]
Chemistry
HgTe bonds are weak. Their enthalpy of formation, around −32kJ/mol, is less than a third of the value for the related compound cadmium telluride. HgTe is easily etched by acids, such as hydrobromic acid.[1][2]
النمو
Bulk growth is from a mercury and tellurium melt in the presence of a high mercury vapour pressure. HgTe can also be grown epitaxially, for example, by sputtering or by metalorganic vapour phase epitaxy.[1][2]
الخصائص الفيزيائية الفريدة

Recently it was shown both theoretically and experimentally, that mercury-telluride quantum well shows a unique new state of matter—the "topological insulator". In this phase, while the bulk is an insulator, current can be carried by electronic states confined close to the sample edges. Unlike the quantum hall effect, here no magnetic field is required to create this unique behavior. In addition, oppositely directed edge states carry opposite spin projections.[5]
مركبات متعلقة
المصادر
- ^ أ ب ت ث Brice, J. and Capper, P. (eds.) (1987) Properties of mercury cadmium telluride, EMIS datareview, INSPEC, IEE, London, UK.
- ^ أ ب ت ث Capper, P. (ed.) (1994) Properties of Narrow-Gap Cadmium-Based Compounds. INSPEC, IEE, London, UK. ISBN 0-85296-880-9
- ^ Boctor, N.Z.; Kullerud, G. (1986). "Mercury selenide stoichiometry and phase relations in the mercury-selenium system". Journal of Solid State Chemistry. 62 (2): 177. Bibcode:1986JSSCh..62..177B. doi:10.1016/0022-4596(86)90229-X.
- ^ Spencer, Joseph; Nesbitt, John; Trewhitt, Harrison; Kashtiban, Reza; Bell, Gavin; Ivanov, Victor; Faulques, Eric; Smith, David (2014). "Raman Spectroscopy of Optical Transitions and Vibrational Energies of ~1 nm HgTe Extreme Nanowires within Single Walled Carbon Nanotubes" (PDF). ACS Nano. 8 (9): 9044–52. doi:10.1021/nn5023632. PMID 25163005.
- ^ König, M; Wiedmann, S; Brüne, C; Roth, A; Buhmann, H; Molenkamp, L. W.; Qi, X. L.; Zhang, S. C. (2007). "Quantum Spin Hall Insulator State in HgTe Quantum Wells". Science. 318 (5851): 766–770. arXiv:0710.0582. Bibcode:2007Sci...318..766K. doi:10.1126/science.1148047. PMID 17885096.
وصلات خارجية
- Thermophysical properties database[dead link] at Germany's Chemistry Information Centre, Berlin