ثلاثي سيلينيد الأنتيمون
| |
| الأسماء | |
|---|---|
أسماء أخرى
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.870 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | Sb2Se3 |
| كتلة مولية | 480.4 g mol-1 |
| المظهر | black crystals |
| الكثافة | 5.81 g/cm3, solid |
| نقطة الانصهار | |
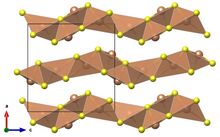
| البنية | |
| البنية البلورية | Orthorhombic, oP20, SpaceGroup = Pnma, No. 62 |
| المخاطر | |
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |
PEL (المسموح)
|
TWA 0.5 mg/m3 (as Sb)[1] |
REL (الموصى به)
|
TWA 0.5 mg/m3 (as Sb)[1] |
| مركبات ذا علاقة | |
أنيونات أخرى
|
antimony(III) oxide, antimony(III) sulfide, antimony(III) telluride |
كاتيونات أخرى
|
arsenic(III) selenide, bismuth(III) selenide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Antimony triselenide is the chemical compound with the formula Sb
2Se
3. The material exists as the sulfosalt mineral antimonselite (IMA symbol: Atm[2]), which crystallizes in an orthorhombic space group.[3] In this compound, antimony has a formal oxidation state +3 and selenium −2. The bonding in this compound has covalent character as evidenced by the black color and semiconducting properties of this and related materials.[4] The low-frequency dielectric constant (ε0) has been measured to be 133 along the c axis of the crystal at room temperature, which is unusually large.[5] Its band gap is 1.18 eV at room temperature.[6]
The compound may be formed by the reaction of antimony with selenium and has a melting point of 885 K.[4]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Applications
Sb
2Se
3 is now being actively explored for application thin-film solar cells.[7] A record light-to-electricity conversion efficiency of 9.2% has been reported.[8]
References
- ^ أ ب NIOSH Pocket Guide to Chemical Hazards 0036
- ^ Warr, L.N. (2021). "IMA-CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Jambor, J. L.; Grew, E. S."New Mineral Names" American Mineralogist, Volume 79, pages 387-391, 1994.
- ^ أ ب Madelung, O (2004). Semiconductors: data handbook (3rd ed.). Springer. ISBN 9783540404880.
- ^ Petzelt, J.; Grigas, J. (January 1973). "Far infrared dielectric dispersion in Sb2S3, Bi2S3 and Sb2Se3 single crystals". Ferroelectrics. 5 (1): 59–68. Bibcode:1973Fer.....5...59P. doi:10.1080/00150197308235780. ISSN 0015-0193.
- ^ Birkett, Max; Linhart, Wojciech M.; Stoner, Jessica; Phillips, Laurie J.; Durose, Ken; Alaria, Jonathan; Major, Jonathan D.; Kudrawiec, Robert; Veal, Tim D. (2018). "Band gap temperature-dependence of close-space sublimation grown Sb2Se3 by photo-reflectance". APL Materials. 6 (8): 084901. doi:10.1063/1.5027157.
- ^ Bosio, Alessio; Foti, Gianluca; Pasini, Stefano; Spoltore, Donato (January 2023). "A Review on the Fundamental Properties of Sb2Se3-Based Thin Film Solar Cells". Energies. 16 (19): 6862. doi:10.3390/en16196862.
- ^ Wong, Lydia Helena; Zakutayev, Andriy; Major, Jonathan Douglas; Hao, Xiaojing; Walsh, Aron; Todorov, Teodor K.; Saucedo, Edgardo (2019). "Emerging inorganic solar cell efficiency tables (Version 1)". J Phys Energy. 1 (3): 032001. Bibcode:2019JPEn....1c2001W. doi:10.1088/2515-7655/ab2338. hdl:10044/1/70500.