حمض التلوريك
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Hexahydroxidotellurium
| |
| أسماء أخرى
Orthotelluric acid, Tellurium(VI) hydroxide
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.334 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
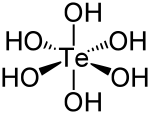
| الصيغة الجزيئية | Te(OH) 6 |
| كتلة مولية | 229.64 g/mol |
| المظهر | White monoclinic crystals |
| الكثافة | 3.07 g/cm3 |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | 50.1 g/100 ml at 30 °C[1] |
| الحموضة (pKa) | 7.68, 11.0 at 18 °C[1] |
| البنية | |
| الشكل الجزيئي | octahedral |
| Dipole moment | 0 D |
| المخاطر | |
| خطر رئيسي | corrosive |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Hydrotelluric acid Tellurous acid Hydrogen telluride |
مركـّبات ذات علاقة
|
Teflic acid Sulfuric acid Selenic acid |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
حمض التلوريك Telluric acid هو مركب كيميائي صيغته Te(OH)
6. وهو صلب أبيض معمول من جزيئات Te(OH)
6 ثمانية الأسطح which persist in aqueous solution.[2] There are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)
6 molecules.[3]
Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water.[3][4] It is used as tellurium-source in the synthesis of oxidation catalysts.[5][6][7]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التحضير
Telluric acid is formed by the oxidation of tellurium or tellurium dioxide with a powerful oxidising agent such as hydrogen peroxide, chromium trioxide or sodium peroxide.[3]
- TeO
2 + H
2O
2 + 2 H
2O → Te(OH)
6
Crystallization of telluric acid solutions below 10 °C gives Te(OH)
6·4H2O.[2]
It is oxidizing, as shown by the electrode potential for the reaction below, although it is kinetically slow in its oxidations.[3]
- H
6TeO
6 + 2 H+
+ 2 e−
⇌ TeO
2 + 4 H
2O, Eo= +1.02 V
Chlorine, by comparison, is +1.36 V and selenous acid is +0.74 V in oxidizing conditions.
الخصائص والتفاعلات
The anhydrous acid is stable in air at 100 °C but above this it dehydrates to form polymetatelluric acid, a white hygroscopic powder (approximate composition (H
2TeO
4)
10), and allotelluric acid, an acid syrup of unknown structure (approximate composition (H
2TeO
4)
3(H
2O)
4).[8][2]
Typical salts of the acid contains the anions [Te(O)(OH)
5]−
and [Te(O)
2(OH)
4]2−. The presence of the tellurate ion TeO2−
4 has been confirmed in the solid state structure of Rb
6[TeO
5][TeO
4].[9]
Strong heating at over 300 °C produces the α crystalline modification of tellurium trioxide, α-TeO
3.
[4] Reaction with diazomethane gives the hexamethyl ester, Te(OCH
3)
6.[2]
Telluric acid and its salts mostly contain hexacoordinate tellurium.[3] This is true even for salts such as magnesium tellurate, MgTeO
4, which is isostructural with magnesium molybdate and contains TeO
6 octahedra.[3]
أشكال أخرى من حمض التلوريك
Metatelluric acid, H
2TeO
4, the tellurium analogue of sulfuric acid, H
2SO
4, is unknown. Allotelluric acid of approximate composition (H
2TeO
4)
3(H
2O)
4, is not well characterised and may be a mixture of Te(OH)
6 and (H
2TeO
4)
n.[2]
أحماض التلوريوم الأخرى
Tellurous acid H
2TeO
3, containing tellurium in its +4 oxidation state, is known but not well characterised.
Hydrogen telluride is an unstable gas that forms hydrotelluric acid upon addition to water.
المراجع
- ^ أ ب Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, ISBN 0-8493-0594-2
- ^ أ ب ت ث ج Greenwood, N. N. (1997). Chemistry of the Elements (2nd Edition ed.). Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ أ ب ت ث ج ح قالب:Cotton&Wilkinson6th
- ^ أ ب Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ "Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid". J. Catal. 285: 48–60. 2012.
- ^ "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol". ACS Catalysis. 3: 1103–1113. 2013.
- ^ Yun, Yang Sik; Lee, Minzae; Sung, Jongbaek; Yun, Danim; Kim, Tae Yong; Park, Hongseok; Lee, Kyung Rok; Song, Chyan Kyung; Kim, Younhwa; Lee, Joongwon; Seo, Young-Jong (2018-12-05). "Promoting effect of cerium on MoVTeNb mixed oxide catalyst for oxidative dehydrogenation of ethane to ethylene". Applied Catalysis B: Environmental (in الإنجليزية). 237: 554–562. doi:10.1016/j.apcatb.2018.06.025. ISSN 0926-3373.
- ^ Loub, J.; Haase, W.; Mergehenn, R. (1979). "Structure of an adduct of orthotelluric acid and urea". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 35 (12): 3039–3041. doi:10.1107/S0567740879011286.
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 526. ISBN 978-0-13-175553-6.
- CS1 errors: unsupported parameter
- CS1 errors: extra text: edition
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- Tellurium compounds
- Hydrogen compounds
- Tellurates
- Oxidizing acids
- عوامل مؤكسدة