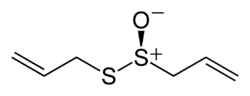
أليسين
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
2-propene-1-sulfinothioic acid S-2-propenyl ester
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.935 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | C6H10OS2 |
| كتلة مولية | 162.28 g/mol |
| الكثافة | 1.112 g/cm3 |
| نقطة الانصهار | |
| نقطة الغليان | |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
الأليسين Allicin ، هو مركب كبريت عضوي يستخرج الثوم، من عائلة الزنبقيات.[1] قام بفصله أول مرة ودراسته في المختبر تشستر ج. كڤاليتو في عام 1944.[2][3] وأليسين هو سائل عديم اللون له رائحة نفاذة مميزة. This compound exhibits antibacterial and anti-fungal properties.[4] Allicin is garlic's defence mechanism against attacks by pests. [5]
التركيب
الفوائد الصحية المحتملة
أثبت الأبحاث فيما بين 1995 و2005 أن يمكن لأليسين: أن يقلل من تصلب الشرايين وترسب الدهون.[6][7], normalize the lipoprotein balance, decrease blood pressure[8][9], have anti-thrombotic[10] and anti-inflammatory activities, and function as an antioxidant to some extent [11][12][13]. Other studies have shown a strong oxidative effect in the gut that can damage intestinal cells.[14] However a randomized clinical trial funded by the National Institutes of Health (NIH) in the United States and published in the Archives of Internal Medicine in 2007 found that the consumption of garlic in any form did not reduce blood cholesterol levels in patients with moderately high baseline cholesterol levels.[15] The fresh garlic used in this study contained substantial levels of allicin so this study casts doubt on the ability of allicin when taken orally to reduce blood cholesterol levels in human subjects.
In 2009, Vaidya, Ingold, and Pratt have clarified exactly how allicin works to produce its medicinal effects, such as trapping damaging radicals. According to them, it is the sulfenic acids, which are produced by the decomposition of allicin, that extremely rapidly react with free radicals, and bind with them. "We suggest that the peroxyl-radical-trapping activity of garlic is primarily due to 2-propenesulfenic acid formed by the decomposition of allicin."[16]
فعاليته كمضاد حيوي
انظر أيضا
- Allyl isothiocyanate، المادة الكيميائية الحريفة الفعالة في المسطردة، radishes, horseradish والواسابي
- Capsaicin، المادة الكيميائية الحريفة الفعالة في فلفل تشيلي
- فلفلين Piperine، المادة الكيميائية الحريفة الفعالة في فلفل أسود
- Syn-propanethial-S-oxide، المادة الكيميائية الموجودة في البصل
- قائمة الكيماويات النباتية في الطعام
| allicin
]].المصادر
- ^ Eric Block (1985). "The chemistry of garlic and onions". Scientific American. 252 (March): 114–119.
- ^ Chester J. Cavallito, John Hays Bailey, "Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action", Journal of the American Chemical Society, 1944, volume 66, pp 1950 - 1951. doi:10.1021/ja01239a048
- ^ Eric Block, "Garlic and Other Alliums: The Lore and the Science" (Cambridge: Royal Society of Chemistry, 2010)
- ^ Chester J. Cavallito, John Hays Bailey "Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action" Journal of the American Chemical Society, 1944, volume 66, pp 1950 - 1951. doi:10.1021/ja01239a048
- ^ http://www.phytochemicals.info/phytochemicals/allicin.php
- ^ S. Eilat, Y. Oestraicher, A. Rabinkov, D. Ohad, D. Mirelman, A. Battler, M. Eldar and Z. Vered (1995). "Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic". Coron. Artery Dis. 6 (12): 985–990. PMID 8723021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ D. Abramovitz, S. Gavri, D. Harats, H. Levkovitz, D. Mirelman, T. Miron, S. Eilat-Adar, A. Rabinkov, M. Wilchek, M. Eldar and Z. Vered, (1999). "Allicin-induced decrease in formation of fatty streaks (atherosclerosis) in mice fed a cholesterol-rich diet". Coron. Artery Dis. 10 (7): 515–519. doi:10.1097/00019501-199910000-00012. PMID 10562920.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Silagy CA, Neil HA (1994). "A meta-analysis of the effect of garlic on blood pressure". J Hypertens. 12(4): 463–468.
- ^ A. Elkayam, D. Mirelman, E. Peleg, M. Wilchek, T. Miron, A. Rabinkov, M. Oron-Herman and T. Rosenthal (2003). "The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats". Am. J. Hypertens. 16 (12): 1053–1056. doi:10.1016/j.amjhyper.2003.07.011. PMID 14643581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Srivastava KC (1986). "Evidence for the mechanism by which garlic inhibits platelet aggregation". Prostaglandins Leukot Med. 22(3): 313–321. doi:10.1016/0262-1746(86)90142-3.
- ^ U. Sela, S. Ganor, I. Hecht, A. Brill, T. Miron, A. Rabinkov, M. Wilchek, D. Mirelman, O. Lider and R. Hershkoviz (2004). "Allicin inhibits SDF-1alpha-induced T cell interactions with fibronectin and endothelial cells by down-regulating cytoskeleton rearrangement, Pyk-2 phosphorylation and VLA-4 expression". Immunology. 111 (4): 391–399. doi:10.1111/j.0019-2805.2004.01841.x. PMC 1782446. PMID 15056375.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lindsey J. Macpherson, Bernhard H. Geierstanger, Veena Viswanath, Michael Bandell, Samer R. Eid, SunWook Hwang, and Ardem Patapoutian (2005). "The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin". Current Biology. 15 (May 24): 929–934.
{{cite journal}}: External link in|title= - ^ Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE and Zygmunt PM (2005). "Pungent products from garlic activate the sensory ion channel TRPA1". Proc Natl Acad Sci USA. 102 (34): 12248–52. doi:10.1073/pnas.0505356102. PMC 1189336. PMID 16103371.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lawson, L. D., Ransom, D. K. and Hughes, B. G. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Throm. Res. 65: 141-156, 1992.
- ^ Gardner CD, Lawson LD, Block E; et al. (2007). "Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial". Arch. Intern. Med. 167 (4): 346–53. doi:10.1001/archinte.167.4.346. PMID 17325296.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vaidya, Vipraja (2009). "Garlic: Source of the Ultimate Antioxidants - Sulfenic Acids". Angewandte Chemie. 121 (1): 163–166. doi:10.1002/ange.200804560. Retrieved 21 Feb 2009.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
وصلات خارجية
- CS1 errors: unsupported parameter
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- كيماويات نباتية
- مركبات الكبريت العضوي
- ألكينات
- عوامل مضادة للالتهاب
- مضادات حيوية
- مضادات أكسدة غذائية
- نكهات نفاذة
- Alliaceae
- أليوم
- ثوم