ثيوسيانات الزئبق الثنائي
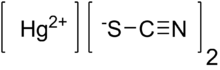
| |
| الأسماء | |
|---|---|
| أسماء أخرى
Mercuric thiocyanate
Mercuric sulfocyanate | |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.008.886 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| الخصائص | |
| الصيغة الجزيئية | Hg(SCN)2 |
| كتلة مولية | 316.755 g/mol |
| المظهر | مسحوق أحادي الميل أبيض |
| الرائحة | عديم الرائحة |
| الكثافة | 3.71 گ/سم³، صلب |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | 0.069 g/100 mL |
| قابلية الذوبان | قابل للذوبان في حمض الهيدروكلوريك المخفف وKCN، أمونيا قليل الذوبان في الكحول، والإيثر |
| المخاطر | |
| NFPA 704 (معيـَّن النار) | |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
46 mg/kg (rat, oral) |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
ثيوسيانات الزئبق الثنائي Mercury(II) thiocyanate (Hg(SCN)2) هو مركب كيميائي غير عضوي، ومعقد تناسقي من Hg2+ وأنيون الثيوسيانات. وهو مسحوق أبيض. ويُنتـِج “ثعبان” ملتوي كبير حين يشتعل، وهو أثر يُعرف بإسم حية فرعون.[1]
التحضير
أول تحضير لثيوسيانات الزئبق ربما تم في 1821 على يد يونس ياكوب برزليوس:
- HgO + 2 HSCN → Hg(SCN)2 + H2O
Evidence for the first pure sample was presented in 1866 prepared by a chemist named Otto Hermes.[1] It is prepared by treating solutions containing mercury(II) and thiocyanate ions. The low solubility product of mercury thiocyanate causes it to precipitate from solution.[2] Most syntheses are achieved by precipitation:
- Hg(NO3)2 + 2 KSCN → Hg(SCN)2 + 2KNO3
حية فرعون
Mercury thiocyanate was formerly used in pyrotechnics causing an effect known as the Pharaoh's serpent or Pharaoh's snake. When the compound is in the presence of a strong enough heat source, a rapid exothermic reaction is started which produces a large mass of coiling serpent-like solid. An inconspicuous flame which is often blue but can also occur in yellow/orange accompanies the combustion. The resulting solid can range from dark graphite grey to light tan in color with the inside generally much darker than the outside.[1]
The reaction has several stages as follows:[3]
Igniting mercury thiocyanate causes it to form an insoluble brown mass that is primarily carbon nitride, C3N4. Mercury sulphide and carbon disulphide are also produced.
2Hg(SCN)2 → 2HgS + CS2 + C3N4
Carbon disulphide combusts to carbon dioxide and sulphur dioxide:
CS2 + 3O2 → CO2 + 2SO2
The heated C3N4 partially breaks down to form nitrogen gas and dicyan:
2C3N4 → 3(CN)2 + N2
Mercury sulphide reacts with oxygen to form mercury vapour and sulphur dioxide. If the reaction is performed inside a container, you will be able to observe a grey film of mercury coating its inner surface.
HgS + O2 → Hg + SO2
This reaction was discovered by Wöhler in 1821, soon after the first synthesis of mercury thiocyanate: "winding out from itself at the same time worm-like processes, to many times its former bulk, a very light material the color of graphite...". For some time, a firework product called "Pharaoschlangen" was available to the public in Germany, but was eventually banned when the toxic properties of the product were discovered through the death of several children mistakenly eating the resulting solid.[1]
A similar, although less extreme, effect to the Pharaoh's serpent can be achieved using a firework known as a black snake. These are generally benign products, usually consisting of sodium bicarbonate or a mixture of linseed oil and naphthalenes.[بحاجة لمصدر]
الاستخدامات
الهامش
- ^ أ ب ت ث Davis, T. L. (1940). "Pyrotechnic Snakes". Journal of Chemical Education. 17 (6): 268–270. doi:10.1021/ed017p268.
- ^ Sekine, T.; Ishii, T. (1970). "Studies of the Liquid-Liquid Partition systems. VIII. The Solvent Extraction of Mercury (II) Chloride, Bromide, Iodide and Thiocyanate with Some Organic Solvents" (pdf). Bulletin of the Chemical Society of Japan. 43 (8): 2422–2429. doi:10.1246/bcsj.43.2422.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Make a Pharaoh's Snake Firework". About.com Education. Retrieved 2016-02-08.
وصلات خارجية
- "Pharaoh's snake". YouTube. September 2, 2008.
