نڤيراپين
 | |
 | |
| البيانات السريرية | |
|---|---|
| فئة السلامة أثناء الحمل |
|
| مسارات الدواء | Oral |
| رمز ATC | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | 93% ± 9% |
| الأيض | Hepatic |
| Elimination half-life | 45 hours |
| الإخراج | Renal: <6% (Parent drug) Biliary <5% (Parent drug) |
| المعرفات | |
| |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.117.250 |
| Chemical and physical data | |
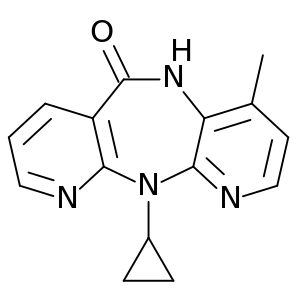
| التركيب | C15H14N4O |
| الكتلة المولية | 266.298 g/mol |
Nevirapine, نيڤيرابين يسوق تحت الأسم التجاري Viramune (Boehringer Ingelheim), is a non-nucleoside reverse transcriptase inhibitor (NNRTI) يستعمل لعلاج عدوىHIV-1 الإيدز.
وكمثل كل أنواع أدوية antiretroviral, فإن الإيدز سرعان ما يبدى مقاومة, إذا أستخدم نيڤيرابين بمفرده, لذا فإن العلاج المقرر يتكون من مجموعة من 3 أنواع أو أكثر من مضادات الفيروس. .
التاريخ
أكتشف نيڤيرابين بواسطة Hargrave et al. at Boehringer Ingelheim Pharmaceuticals, Inc. , واحدة من Boehringer Ingelheim مجموعة شركات . ويتم تغطيته بواسطة براءة أميريكية رقم 5,366,972 وببراءات أجنبية مماثلة. نيڤيرابين كان أول NNRTI يقر بواسطةFood and Drug Administration (FDA) في الولايات المتحدة الأميريكية. تم الموافقة عليه 21 يونيو, 1996 للبالغين سبتمبر 11, 1998 وللأطفال. و أجيز في أوروبا عام 1997.
طريقة العمل
نيڤيرابينيفشل في مجموعة non-nucleoside reverse transcriptase inhibitor (NNRTI) مضادات الفيروس. Both nucleoside and non-nucleoside RTIs تثبط نفس الهدف , أنزيم reverse transcriptase , وهو أنزيم فعال يمتلكه الفيروس ويقوم بنسخ الرنا الفيروسي الى دنا . وليس كمثل nucleoside RTIs , الذى يلتصق بالجزء الحيوى من , NNRTIs يلتصق بالفيروس داخل جيب يسمى جيب NNRTI.
نيڤيرابين ليس فعالا ضد HIV-2, حيث أن جيبHIV-2 يعاكس transcriptase الذى يمتلك تركيبا مختلفا , ويبدى مقاومة داخلية الى NNRTI class.[1]
المقاومة لنيڤيرابين تتكون سريعا, أذا لم يتم إحباط عملية نسخ دنا الفيروس سريعا .[2] The most common mutations observed after nevirapine treatment are Y181C and K103N, which are also observed with other NNRTIs.[3][4] As all NNRTIs bind within the same pocket, viral strains which are resistant to nevirapine are usually also resistant to the other NNRTIs, efavirenz and delavirdine.
الفعالية السريرية
Nevirapine in triple combination therapy has been shown to suppress viral load effectively when used as initial antiretroviral therapy (i.e., in antiretroviral-naive patients).[2] Some clinical trials have demonstrated comparable HIV suppression with nevirapine-based regimens to that achieved with protease inhibitors (PIs)[5][6] or efavirenz.[7] Although concerns have been raised about nevirapine-based regimens in those starting therapy with high viral load or low CD4 count, some analyses suggest that nevirapine may be effective in these patients.[7]
Nevirapine may also form a useful component of salvage regimens after virological failure, usually in combination with one or more PIs as well as nRTIs, especially in those who have not previously taken an NNRTI.
الآثار الجانبية
The most common adverse effect of nevirapine is the development of mild or moderate rash (13%).[3][8] Severe or life-threatening skin reactions have been observed in 1.5% of patients, including Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity.[3]
Nevirapine may cause severe or life-threatening liver toxicity, usually emerging in the first six weeks of treatment.[3][9] In 2000, the U.S. Food and Drug Administration issued a black box label on nevirapine, warning that it could cause severe liver damage, including liver failure.[10] Unacceptably high risk of serious liver symptoms in certain patient groups (women with CD4 count >250 and men >400)[7] has led the U.S. DHSS to recommend the restriction of nevirapine use to those at lower risk, unless the benefit to the patient clearly outweighs the risk;[9] although in the 2NN study which found these CD4 limits, the effect was seen only in patients recruited from Thailand. More recent studies on the use of Nevirapine in people with higher CD4 cell counts have come to the following conclusion: Treatment-experienced patients who start NVP-based combination therapy with low pre–ART and high current CD4 cell counts and an undetectable VL have a similar likelihood for discontinuing NVP therapy because of hypersensitivity reactions (HSRs), compared with treatment-naive patients with low CD4 cell counts. This suggests that NVP-based combination therapy may be safely initiated in such patients. However, in similar patients with a detectable VL, it is prudent to continue to adhere to current CD4 cell count thresholds. [11] The U.S. Public Health Service Task Force advocates caution in the use of nevirapine in pregnancy due to toxicity issues, which may be exacerbated during pregnancy.[12]
التفاعلات الدوائية
Significant lowering of nevirapine levels occurs with the anti-tuberculosis drug, rifampicin, and the drugs should not be administered together.[3]
Nevirapine is an inducer of cytochrome P450 isoenzymes CYP3A4 and CYP2B6. It reduces the levels of several co-administered drugs including the antiretrovirals efavirenz, indinavir, lopinavir, nelfinavir and saquinavir, as well as clarithromycin, ketoconazole, forms of hormonal contraception, and methadone.[3]
منع إنتقال العدوى من الأم الى الطفل
A single dose of nevirapine given to both mother and child reduced the rate of HIV transmission by almost 50% compared with a very short course of zidovudine (AZT) prophylaxis, in a clinical trial in Uganda.[13] A subsequent study in تايلند showed that prophylaxis with single-dose nevirapine in addition to zidovudine is more effective than zidovudine alone.[14] These and other trials have led the World Health Organization to endorse the use of single-dose nevirapine prophylaxis in many developing world settings as a cost-effective way of reducing mother-to-child transmission. However, in the United States the Ugandan study was deemed flawed [15] and as of 2006 the FDA has not approved of such nevirapine prophylaxis.[16] Another clinical trial, Using Nevirapine to Prevent Mother-to-Child HIV Transmission During Breastfeeding is scheduled for completion in March 2011.[17]
A major concern with this approach is that NNRTI resistance mutations are commonly observed in both mothers and infants after single-dose nevirapine,[18] and may compromise the response to future NNRTI-containing regimens.[19] A short course of maternal zidovudine/lamivudine is recommended by the U.S. Public Health Service Task Force to reduce this risk.[12]
جدل في أفريقيا
U.S. President George W. Bush's $500 million plan to help combat the African AIDS epidemic includes nevirapine, among other medications and programs. Nevirapine has also been tested in trials in Africa, some of which have been highly controversial.
South African President Thabo Mbeki accused the الولايات المتحدة of using Africans as "guinea pigs".[20] Questions regarding the efficacy of the antiretroviral nevirapine when compared with its side effects were the main stated reason for President Mbeki's concern. Until recently, however, Mbeki endorsed claims by some scientists that HIV is not the cause of AIDS—findings which are considered well outside the realm of reasonable scientific thought by the vast majority of the scientific community.[21]
المراجع
- ^ Ren J, Bird LE, Chamberlain PP, Stewart-Jones GB, Stuart DI, Stammers DK (2002). "Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors". Proc Natl Acad Sci USA. 99 (22): 14410–5. doi:10.1073/pnas.222366699. PMC 137897. PMID 12386343.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Montaner JS, Reiss P, Cooper D; et al. (1998). "A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study". JAMA. 279 (12): 930–7. doi:10.1001/jama.279.12.930. PMID 9544767.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث ج ح Viramune (nevirapine) tablets; Viramune (nevirapine) oral suspension prescribing information
- ^ Conway B, Wainberg MA, Hall D; et al. (2001). "Development of drug resistance in patients receiving combinations of zidovudine, didanosine and nevirapine". AIDS. 15 (10): 1269–74. doi:10.1097/00002030-200107060-00008. PMID 11426071.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ van Leeuwen R, Katlama C, Murphy RL; et al. (2003). "A randomized trial to study first-line combination therapy with or without a protease inhibitor in HIV-1-infected patients". AIDS. 17 (7): 987–99. doi:10.1097/00002030-200305020-00007. PMID 12700448.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Podzamczer D, Ferrer E, Consiglio E; et al. "A randomized clinical trial comparing nelfinavir or nevirapine associated to zidovudine/lamivudine in HIV-infected naive patients (the Combine Study)". Antiviral Ther. 7: 81–90.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ أ ب ت van Leth F, Andrews S, Grinsztejn B; et al. (2005). "The effect of baseline CD4 cell count and HIV-1 viral load on the efficacy and safety of nevirapine or efavirenz-based first-line HAART". AIDS. 19 (5): 463–71. PMID 15764851.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Facts sheet from the AIDS Treatment Data Network". Retrieved 2006-01-16.
- ^ أ ب DHHS panel. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (May 4, 2006). (Available for download from AIDSInfo)
- ^ Viramune (nevirapine) letter (November 2000)
- ^ Wit FW, Kesselring AM, Gras L; et al. (2008). "Discontinuation of nevirapine because of hypersensitivity reactions in patients with prior treatment experience, compared with treatment-naive patients: the ATHENA cohort study". Clin Infect Dis. 46 (6): 933–40. doi:10.1086/528861. PMID 18271750.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. Public Health Service Task Force. (November 17, 2005) (Available for download from AIDSInfo)
- ^ Guay LA, Musoke P, Fleming T; et al. (1999). "Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial". Lancet. 354 (9181): 795–802. doi:10.1016/S0140-6736(99)80008-7. PMID 10485720.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lallemant M, Gonzague Jourdain G, Sophie Le Coeur S, et al. (2004) Single-Dose Perinatal Nevirapine plus Standard Zidovudine to Prevent Mother-to-Child Transmission of HIV-1 in Thailand. N Engl J Med 351: 217-28
- ^ The HIVNET 012 Study and the Safety and Effectiveness of Nevirapine in Preventing Mother-to-Infant Transmission of HIV, http://www3.niaid.nih.gov/news/newsreleases/2004/hivnet012.htm
- ^ Celia Farber, "Out of Control: AIDS and the Corruption of Science" http://www.harpers.org/archive/2006/03/0080961
- ^ http://clinicaltrials.gov/show/NCT00074412
- ^ Johnson JA, Li JF, Morris L; et al. (2005). "Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated". J Infect Dis. 192 (1): 16–23. doi:10.1086/430741. PMID 15942889.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jourdain G, Ngo-Giang-Huong N, Le Coeur S; et al. (2004). "Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy". N Engl J Med. 351 (3): 229–40. doi:10.1056/NEJMoa041305. PMID 15247339.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Nevirapine Controversy". Retrieved 2006-01-16.
- ^ "AEGiS-M&G: Mbeki's Aids Letter Defies Belief". Retrieved 2006-04-12.
- CS1 errors: unsupported parameter
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Non-nucleoside reverse transcriptase inhibitors
- الأدوية الأساسية حسب منظمة الصحة العالمية
- أمراض