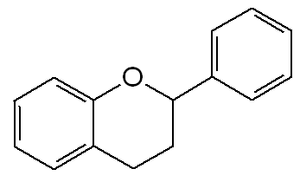
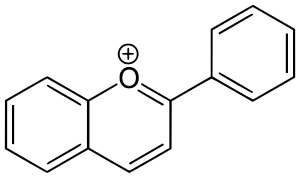
فلاڤونويد
الفلاڤونويد Flavonoids (أو باي فلاڤونويدات) ويعرف أيضا باسم ڤيتامين پ[1] ، هي مجموعة مستقلبات ثانوية نباتية أو أصباغ صفراء لها بنية مشابهة للفلاڤونات. حسب تسمية الاتحاد الدولي للكيمياء البحتة والتطبيقية،[2] يمكن تصنيف الفلاڤونويدات كالتالي:
- فلاڤونويدات، derived from 2-phenylكرومن-4-وان (2-phenyl-1,4-benzopyrone) structure (examples: quercetin, rutin).
- أيزوفلاڤونويدات، derived from 3-phenylكرومن-4-وان (3-phenyl-1,4-benzopyrone) structure
- نيوفلاڤونويدات, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure.
التخليق الحيوي
وظائف الفلاڤونويدات في النبات
ينتشر الفلاڤونويد على نطاق واسع في النباتات ويقوم بالكثير من الوظائف. وتعتبر الفلاڤونويدات من أهم الصبغات الحيوية اللازمة لتلوين الأزهار حيث ينتج اللون الأصفر أو الأحمر/الأزرق في البتلات والتي تعمل على جذب الحيوانات الملقحة.
الفوائد الصحية المحتملة للإنسان
Flavonoids (specifically flavanoids such as the catechins) are "the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants".[3] Flavonols, the original bioflavonoids such as quercetin, are also found ubiquitously, but in lesser quantities.
The widespread distribution of flavonoids, their variety and their relatively low toxicity compared to other active plant compounds (for instance alkaloids) mean that many animals, including humans, ingest significant quantities in their diet. Preliminary research indicates that flavonoids may modify allergens, viruses, and carcinogens, and so may be biological "response modifiers". In vitro studies show that flavonoids also have anti-allergic, anti-inflammatory,[4] anti-microbial,[5][6] anti-cancer,[7] and anti-diarrheal activities.[8]
كمضاد الأكسدة
Flavonoids (both flavonols and flavanols) are most commonly known for their antioxidant activity. Additionally, at high experimental concentrations that would not exist in vivo, the antioxidant abilities of flavonoids in vitro are stronger than those of vitamin C and E.[9]
Consumers and food manufacturers have become interested in flavonoids for their possible medicinal properties, especially their putative role in prevention of cancers and cardiovascular diseases. Although physiological evidence is not yet established, the beneficial effects of fruits, vegetables, tea, and red wine have sometimes been attributed to flavonoid compounds.
Alternatively, research conducted at the Linus Pauling Institute and evaluated by the European Food Safety Authority indicates that, following dietary intake, flavonoids themselves are of little or no direct antioxidant value.[10][11][12] As body conditions are unlike controlled test tube conditions, flavonoids and other polyphenols are poorly absorbed (less than 5%), with most of what is absorbed being quickly metabolized and excreted.
The increase in antioxidant capacity of blood seen after the consumption of flavonoid-rich foods is not caused directly by flavonoids themselves, but most likely is due to increased uric acid levels that result from metabolism of flavonoids.[13] According to Frei, "we can now follow the activity of flavonoids in the body, and one thing that is clear is that the body sees them as foreign compounds and is trying to get rid of them."
مقاومة السرطان
Flavonoids might induce mechanisms that affect cancer cells and inhibit tumor invasion.[13] In preliminary studies, UCLA cancer researchers proposed that smokers who ate foods containing certain flavonoids, such as catechins found in strawberries and green and black teas, kaempferol from brussel sprouts and apples, and quercetin from beans, onions and apples, may have reduced risk of obtaining lung cancer.[14]
الآثار الصحية الضارة للإنسان
مسبب محتمل للسرطان
Flavonoids were found to be strong topoisomerase inhibitors and induce DNA mutations in the MLL gene, which are common findings in neonatal acute leukemia.[15][16] The DNA changes were increased by treatment with flavonoids in cultured blood stem cells.[17] A high flavonoid-content diet in mothers is suspected to increase risk particularly of acute myeloid leukemia in neonates.[18][19][20]
Polyphenols (flavonoids in one set of experiments and delphinidin in another[21]) were found to be strong topoisomerase inhibitors, similar to some chemotherapeutic anticancer drugs including etoposide and doxorubicin.[22] This property may be responsible for both an anticarcinogenic-proapoptotic effect and a carcinogenic, DNA damaging potential of the substances.
أمثلة على الفلاڤونويدات
كيرستين
إپيكاتچين
المصادر الغذائية
الحمضيات

الشاي
النبيذ
الشيكولاتة الداكنة
المجموعات الفرعية
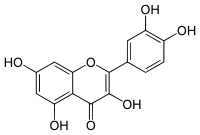
الفلاڤونات
تنقسم الفلاڤوناتإلى أربع مجموعات:[23]
| المجموعة | الهيكل | أمثلة | |||
|---|---|---|---|---|---|
| الوصف | المجموعات الوظيفية | الصيغة الهيكلية | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavone | 2-phenylchromen-4-one | ✗ | ✗ | 
|
Luteolin, Apigenin, Tangeritin |
| Flavonol or 3-hydroxyflavone |
3-hydroxy-2-phenylchromen-4-one | ✓ | ✗ | 
|
Quercetin, Kaempferol, Myricetin, Fisetin, Isorhamnetin, Pachypodol, Rhamnazin |
| Flavanone | 2,3-dihydro-2-phenylchromen-4-one | ✗ | ✓ | 
|
Hesperetin, Naringenin, Eriodictyol, Homoeriodictyol |
| Flavanonol or 3-Hydroxyflavanone or 2,3-dihydroflavonol |
3-hydroxy-2,3-dihydro-2-phenylchromen-4-one | ✓ | ✓ | 
|
Taxifolin (or Dihydroquercetin), Dihydrokaempferol |
إيزوفلاڤونات
فلاڤون-3-ols, Flavan-4-ols, Flavan-3,4-diols, and proanthocyanidins
Derivatives of flavan.
| Skeleton | Name |
|---|---|

|
Flavan-3-ol |

|
Flavan-4-ol |

|
Flavan-3,4-diol (leucoanthocyanidin) |
- Flavan-3-ols (also known as flavanols) and Proanthocyanidins
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton.
- Catechins (Catechin (C), Gallocatechin (GC), Catechin 3-gallate (Cg), Gallocatechin 3-gallate (GCg)), Epicatechins (Epicatechin (EC), Epigallocatechin (EGC), Epicatechin 3-gallate (ECg), Epigallocatechin 3-gallate (EGCg))
- Proanthocyanidins are dimers, trimers, oligomers, or polymers of the flavanols.
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton.
أنثوسيانيدينات
- أنثوسيانيدينات
- Anthocyanidins are the aglycones of anthocyanins. Anthocyanidins use the flavylium (2-phenylchromenylium) ion skeleton
- Examples: Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin
تواجده في الكائنات الدقيقة
انظر أيضا
المصادر
- ^ vitamin P, dictionary results
- ^ Flavonoids (isoflavonoids and neoflavonoids)., IUPAC Compendium of Chemical Terminology
- ^ Spencer, Jeremy P. E. (2008). "Flavonoids: modulators of brain function?". British Journal of Nutrition. 99: ES60–77. doi:10.1017/S0007114508965776. PMID 18503736.
- ^ "Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer". Yamamoto and Gaynor 107 (2): 135 -- Journal of Clinical Investigation.
- ^ Cushnie TPT, Lamb AJ (2005). "Antimicrobial activity of flavonoids". International Journal of Antimicrobial Agents. 26 (5): 343–356. doi:10.1016/j.ijantimicag.2005.09.002. PMID 16323269.
- ^ Cushnie TPT, Lamb AJ (2011). "Recent advances in understanding the antibacterial properties of flavonoids". International Journal of Antimicrobial Agents. 38 (2): 99–107. doi:10.1016/j.ijantimicag.2011.02.014. PMID 21514796.
- ^ de Sousa RR, Queiroz KC, Souza AC, Gurgueira SA, Augusto AC, Miranda MA, Peppelenbosch MP, Ferreira CV, Aoyama H. (2007). "Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin". J Enzyme Inhib Med Chem. 22 (4): 439–444. doi:10.1080/14756360601162063. PMID 17847710.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schuier M, Sies H, Illek B, Fischer H (1 October 2005). "Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia". J. Nutr. 135 (10): 2320–5. PMID 16177189.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bagchi Manashi, Mark Milnes, Casey Williams, Jaya Balmoori, Xumei Ye, Sidney Stohs and Debasis Bagchi (1999). "Acute and chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract". Nutrition Research. 19 (8): 1189–1199. doi:10.1016/S0271-5317(99)00080-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lotito SB, Frei B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon?". Free Radic. Biol. Med. 41 (12): 1727–46. doi:10.1016/j.freeradbiomed.2006.04.033. PMID 17157175.
- ^ Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061, EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3 European Food Safety Authority (EFSA), Parma, Italy, EFSA Journal 2010; 8(2):1489
- ^ Williams RJ, Spencer JP, Rice-Evans C (2004). "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine. 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب "Studies force new view on biology of flavonoids", by David Stauth, EurekAlert!. Adapted from a news release issued by Oregon State University. URL accessed
- ^ UCLA news May 2008 - Fruits, vegetables, teas may protect smokers from lung cancer
- ^ Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H; et al. (1993). "Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations". N Engl J Med. 329 (13): 909–14. doi:10.1056/NEJM199309233291302. PMID 8361504.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD (2000). "Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia". Proc Natl Acad Sci U S A. 97 (9): 4790–5. doi:10.1073/pnas.070061297. PMC 18311. PMID 10758153.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barjesteh van Waalwijk van Doorn-Khosrovani S, Janssen J, Maas LM, Godschalk RW, Nijhuis JG, van Schooten FJ (2007). "Dietary flavonoids induce MLL translocations in primary human CD34+ cells". Carcinogenesis. 28 (8): 1703–9. doi:10.1093/carcin/bgm102. PMID 17468513.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ross JA (1998). "Maternal diet and infant leukemia: a role for DNA topoisomerase II inhibitors?". Int J Cancer Suppl. 11: 26–8. PMID 9876473.
- ^ Ross JA (2000). "Dietary flavonoids and the MLL gene: A pathway to infant leukemia?". Proc Natl Acad Sci U S A. 97 (9): 4411–3. doi:10.1073/pnas.97.9.4411. PMC 34309. PMID 10781030.
- ^ Spector LG, Xie Y, Robison LL, Heerema NA, Hilden JM, Lange B; et al. (2005). "Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children's oncology group". Cancer Epidemiol Biomarkers Prev. 14 (3): 651–5. doi:10.1158/1055-9965.EPI-04-0602. PMID 15767345.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Delphinidin Modulates the DNA-Damaging Properties of Topoisomerase II Poisons. Esselen, Jessica Fritz, Melanie Hutter and Doris Marko, Chem. Res. Toxicol., 2009, 22 (3), pp 554–564 doi:10.1021/tx800293v
- ^ Bandele, O.J.; Clawson, S.J.; Osheroff, N. (2008). "Dietary polyphenols as topoisomerase II poisons: B-ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement". Chemical Research in Toxicology (6): 1253–1260
{{cite journal}}: CS1 maint: postscript (link)doi:10.1021/tx8000785 - ^ Phenolics:figure 4