ايزو ستير حيوي
في الكيمياء الدوائية فإن الأيزوستيرات الحيوية bioisosteres هي متبادلات أو مجموعات ذات صفات فيزيائية أو كيميائية متشابهة، والتي تنقل صفات حيوية مشابهة إلى مركب كيميائي. وفي تصميم الأدوية، فإن الغاية من تبديل أحد الأيزو ستيرات بآخر يكون لتحسين الصفات الحيوية أو الكيميائية المطلوبة لمركب دون احداث تغيرات مهمة في البنية الكيميائية. الاستخدام الأساسي لهذا المصطلح وتقنياته يتعلق بالعلوم الصيدلانية.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
أمثلة
الآيزو ستيرات الحيوية الكلاسيكية
فعلى سبيل المثال، استبدال ذرة هيدروجين بذرة فلور في موقع أكسدة أيضية في a drug candidate may prevent such metabolism from taking place. Because the fluorine atom is similar in size to the hydrogen atom the overall topology of the molecule is not significantly affected, leaving the desired biological activity unaffected. However, with a blocked pathway for metabolism, the drug candidate may have a longer half-life.
- Procainamide, an amide, has a longer duration of action than Procaine, an ester, because of the isosteric replacement of the ester oxygen with a nitrogen atom.[1] Procainamide is a classical bioisostere because the valence electron structure of a disubstituted oxygen atom is the same as a trisubstituted nitrogen atom, as Langmuir showed.
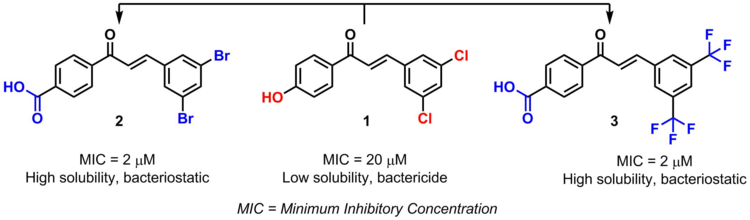
Another example are chalcones bioisosteres. By modifying certain substituents, the pharmacological activity of the chalcone and its toxicity are also modified.[2]
الآيزو ستيرات الحيوية غير الكلاسيكية
Non-classical bioisosteres may differ in a multitude of ways from classical bioisosteres, but retain the focus on providing similar sterics and electronic profile to the original functional group. Whereas classical bioisosteres commonly conserve much of the same structural properties, nonclassical bioisosteres are much more dependent on the specific binding needs of the ligand in question and may substitute a linear functional group for a cyclic moiety, an alkyl group for a complex heteroatom moiety, or other changes that go far beyond a simple atom-for-atom switch.
For example, a chlorine -Cl group may often be replaced by a trifluoromethyl -CF3 group, or by a cyano -C≡N group, but depending on the particular molecule used the substitution may result in little change in activity, or either increase or decrease affinity or efficacy depending on what factors are important for ligand binding to the target protein. Another example is aromatic rings, a phenyl -C6H5 ring can often be replaced by a different aromatic ring such as thiophene or naphthalene which may improve efficacy, change specificity of binding, or reduce metabolically labile sites on the molecule, resulting in better pharmacokinetic properties.
- Alloxanthine is an inhibitor of xanthine oxidase. It is also an isostere of xanthine, the normal substrate for the enzyme.[3] Alloxanthine is considered a non-classical bioisostere because of the scaffold change.
- Silafluofen is an organosilicon analogue of pyrethroid insecticide Etofenprox, wherein a carbon center has been replaced by isosteric silicon, and in addition, one hydrogen atom is replaced by isosteric fluorine atom.[4]
انظر أيضاً
المصادر
- Annual Reports in Medicinal Chemistry, Vol. 21 (1986), 283-291.
- ^ Comprehensive Pharmacy Review, 6th edition, Leon Shargel, Alan H. Mutnick, p.264
- ^ Gomes, Marcelo N. (2017). "Chalcone Derivatives: Promising Starting Points for Drug Design". Molecules. 22 (8): 1210. doi:10.3390/molecules22081210. PMC 6152227. PMID 28757583.
- ^ Comprehensive Pharmacy Review, 6th edition, Leon Shargel, Alan H. Mutnick, p.264
- ^ Showell, G. A.; Mills, J. S. (2003). "Chemistry Challenges in Lead Optimization: Silicon Isosteres in Drug Discovery". Drug Discovery Today. 8 (12): 551–556. doi:10.1016/S1359-6446(03)02726-0. PMID 12821303.