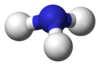
فوسفين

| |||
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك
Phosphane
| |||
| أسماء أخرى
Phosphamine
Phosphorus trihydride Phosphorated hydrogen | |||
| المُعرِّفات | |||
| رقم CAS | |||
| ECHA InfoCard | 100.029.328 | ||
| رقم EC |
| ||
PubChem CID
|
|||
| رقم RTECS |
| ||
| UN number | 2199 | ||
CompTox Dashboard (EPA)
|
|||
| الخصائص | |||
| الصيغة الجزيئية | PH3 | ||
| كتلة مولية | 33.99758 g/mol | ||
| المظهر | colorless gas | ||
| الكثافة | 1.379 g/l, gas (25 °C) | ||
| نقطة الانصهار | |||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | 31.2 mg/100 ml (17 °C) | ||
| اللزوجة | 1.1 x 10−5 Pa s | ||
| البنية | |||
| الشكل الجزيئي | Trigonal pyramidal | ||
| Dipole moment | 0.58 D | ||
| الكيمياء الحرارية | |||
| الإنتالپية المعيارية للتشكل ΔfH |
+22.89 kJ/mol | ||
| المخاطر | |||
تبويب الاتحاد الاوروپي (DSD)
|
قابل للاشتعال بشدة (F+) سام جداً (T+) أكال (C) خطير على البيئة (N) | ||
| توصيف المخاطر | R12, R17, R26, R34, R50 | ||
| تحذيرات وقائية | (S1/2), S28, S36/37, S45, S61, S63 | ||
| NFPA 704 (معيـَّن النار) | |||
| نقطة الوميض | غاز قابل للاشتعال | ||
| حدود الانفجار | 1.8% – ؟ | ||
| مركبات ذا علاقة | |||
كاتيونات أخرى
|
أمونيا Arsine Stibine Bismuthine | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
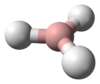
فوسفين Phosphine (اسم أيوپاك: phosphane) هو مركب ذو صيغة كيميائية PH3. وهو غاز عديم اللون، قابل للاشتعال، وسام. الفوسفين النقي هو عديم الرائحة، ولكن عينات الدرجة التقنية تكون ذات رائحة كريهة مثل الثوم أو السمك المنتن، بسبب وجود الفوسفين المـُعـّوَّض و دايفوسفين (P2H4). الفوسفينات هم أيضاً مجموعة من مركبات فسفور عضوي بالصيغة R3P (R = مشتقة عضوية). الفوسفينات العضوية مهمة في المحفزات حيث يتعقدوا إلى أيونات فلزية؛ المعقدات المشتقة من الفوسفين المتماكب يمكنها تحفيز التفاعلات ليعطي منتجات متماكبة .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
البنية والخصائص
PH3 is a trigonal pyramidal molecule with C3v molecular symmetry. The length of the P-H bond is 1.42 Å, the H-P-H bond angles are 93.5°. The dipole moment is 0.58 D, which increases with substitution of methyl groups in the series: CH3PH2, 1.10 D; (CH3)2PH, 1.23 D; (CH3)3P, 1.19 D. In contrast, the dipole moments of amines decrease with substitution, starting with ammonia, which has a dipole moment of 1.47 D. The low dipole moment and almost orthogonal bond angles lead to the conclusion that in PH3 the P-H bonds are almost entirely pσ(P) – sσ(H) and phosphorus 3s orbital contributes little to the bonding between phosphorus and hydrogen in this molecule. For this reason, the lone pair on phosphorus may be regarded as predominantly formed by the 3s orbital of phosphorus. The upfield chemical shift of the phosphorus atom in the 31P NMR spectrum accords with the conclusion that the lone pair electrons occupy the 3s orbital (Fluck, 1973). This electronic structure leads to a lack of nucleophilicity in general and lack of basicity in particular (pKaH = –14),[1] as well as an ability to form only weak hydrogen bonds.[2]
The aqueous solubility of PH3 is slight; 0.22 mL of gas dissolve in 1 mL of water. Phosphine dissolves more readily in non-polar solvents than in water because of the non-polar P-H bonds. It is technically amphoteric in water, but acid and base activity is poor. Proton exchange proceeds via a phosphonium (PH4+) ion in acidic solutions and via PH2− at high pH, with equilibrium constants Kb = 4 × 10−28 and Ka = 41.6 × 10−29.
Phosphine burns producing a dense white cloud of phosphoric acid:
- PH3 + 2 O2 → H3PO4
التحضير والتواجد
Phosphine may be prepared in a variety of ways.[3] Industrially it can be made by the reaction of white phosphorus with sodium or potassium hydroxide, producing potassium or sodium hypophosphite as a by-product.
- 3 KOH + P4 + 3 H2O → 3 KH2PO2 + PH3
Alternatively the acid-catalyzed disproportioning of white phosphorus yields phosphoric acid and phosphine. Both routes have industrial significance; the acid route is the preferred method if further reaction of the phosphine to substituted phosphines is needed. The acid route requires purification and pressurizing. It can also be made (as described above) by the hydrolysis of a metal phosphide, such as aluminium phosphide or calcium phosphide. Pure samples of phosphine, free from P2H4, may be prepared using the action of potassium hydroxide on phosphonium iodide (PH4I).
السبل المعملية
It is prepared in the laboratory by disproportionation of phosphorous acid[4]
- 4 H3PO3 → PH3 + 3 H3PO4
Phosphine evolution occurs at around 200 °C. Alternative methods involve the hydrolysis of aluminium phosphide, calcium phosphide, and tris(trimethylsilyl)phosphine.
التواجد
Phosphine is a constituent of the Earth's atmosphere at very low and highly variable concentrations.[5] It may contribute significantly to the global phosphorus biochemical cycle. The most likely source is reduction of phosphate in decaying organic matter, possibly via partial reductions and disproportionations, since environmental systems do not have known reducing agents of sufficient strength to directly convert phosphate to phosphine.[6]
It is also found in Jupiter's turbulent atmosphere, where it forms in the planet's hot interior and reacts with other compounds in the upper atmosphere.[7] The abiotic synthesis of phosphine takes enormous amounts of energy, such as in the planet-sized convective storms of gas giants.[8] Phosphine has also been detected in the atmosphere of Venus, and its origin is currently unexplained. The paper announcing the discovery suggests that the phosphine "could originate from unknown photochemistry or geochemistry, or, by analogy with biological production of PH3 on Earth, from the presence of life".[9][10][11] Venus lacks the energy to form phosphine the way Jupiter does, requiring an alternate explanation for its presence.[11]
الفوسفينات
Organophosphines are compounds with the formula PRnH3−n. These compounds are often classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Their reactivity is also similar – they can be oxidized to the phosphorus(V) level, they can be protonated and alkylated at phosphorus to give phosphonium salts, and, for primary and secondary derivatives, they can be deprotonated by strong bases to give organophosphide derivatives.
الفوسفينات الأولية
Primary phosphines are typically prepared by alkylation of phosphine. Simple alkyl derivatives such as methylphosphine (CH3PH2) are prepared by alkylation of alkali metal derivatives MPH2 (M = Li, Na, K). Another synthetic route involves treatment of the corresponding chlorophosphines with hydride reagents. For example, reduction of dichlorophenylphosphine with lithium aluminium hydride affords phenylphosphine (PhPH2).
الفوسفينات الثانوية
Secondary phosphines are prepared analogously to the primary phosphines. They are also obtained by alkali-metal reductive cleavage of triarylphosphines followed by hydrolysis of the resulting phosphide salt. The latter route is employed to prepare diphenylphosphine (Ph2PH). Diorganophosphinic acids, R2P(O)OH, can also be reduced with diisobutylaluminium hydride. Secondary phosphines are typically protic in character. But when modified with suitable substituents, as in certain (rare) diazaphospholenes (scheme 3), the polarity of the P-H bond can be inverted (see: umpolung) and the resulting phosphine hydride can reduce a carbonyl group as in the example of benzophenone in yet another way.[12]
الفوسفينات الثالثية
Tertiary phosphines are generally obtained by treatment of phosphorus trichloride or triphenylphosphite with organolithium reagents or Grignard reagents. They are commonly used as ligands in coordination chemistry. Tertiary phosphines of the type PRR'R" are "P-chiral" and optically stable.
الفوسفينات الحلقية
Secondary and tertiary phosphines occur in cyclic forms. Three-membered rings are phosphiranes (unsaturated: phosphirenes), five-membered rings are phospholanes (unsaturated: phosphole), and six-membered rings are phosphinanes.
الاستخدامات
كيمياء الفسفور العضوي
Phosphine is mainly consumed as an intermediate in organophosphorus chemistry. In an illustrative reaction, formaldehyde adds in the presence of hydrogen chloride to give tetrakis(hydroxymethyl)phosphonium chloride, which is used in textiles. The hydrophosphination of alkenes is versatile route to a variety of phosphines. For example in the presence of basic catalysts PH3 adds of Michael acceptors such as acrylonitrile:[13]
- PH3 + 3 CH2=CHZ → P(CH2CH2Z)3 (Z = NO2, CN, C(O)NH2)
Acid catalysis is applicable to hydrophosphination with isobutylene and related analogues:
- PH3 + R2C=CH2 → R2(CH3)CPH2 (R = Me, alkyl, etc)
الإلكترونيات الدقيقة
Phosphine is used as a dopant in the semiconductor industry, and a precursor for the deposition of compound semiconductors. Commercially significant products include gallium phosphide and indium phosphide.[14]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
مبخـِّر
For farm use, pellets of aluminium phosphide, calcium phosphide, or zinc phosphide release phosphine upon contact with atmospheric water or rodents' stomach acid. These pellets also contain agents to reduce the potential for ignition or explosion of the released phosphine. A more recent alternative is the use of phosphine gas itself which requires dilution with either CO2 or N2 or even air to bring it below the flammability point. Use of the gas avoids the issues related with the solid residues left by metal phosphide and results in faster, more efficient control of the target pests.
Because the previously popular fumigant methyl bromide has been phased out in some countries under the Montreal Protocol, phosphine is the only widely used, cost-effective, rapidly acting fumigant that does not leave residues on the stored product. Pests with high levels of resistance toward phosphine have become common in Asia, Australia and Brazil. High level resistance is also likely to occur in other regions, but has not been as closely monitored. Genetic variants that contribute to high level resistance to phosphine have been identified in the dihydrolipoamide dehydrogenase gene.[15] Identification of this gene now allows rapid molecular identification of resistant insects.
السلامة
Phosphine gas is denser than air and hence may collect in low-lying areas. It can form explosive mixtures with air, and may also self-ignite.
Phosphine can be absorbed into the body by inhalation. Direct contact with phosphine liquid – although unlikely to occur – may cause frostbite, like other cryogenic liquids. The main target organ of phosphine gas is the respiratory tract.[16] According to the 2009 U.S. National Institute for Occupational Safety and Health (NIOSH) pocket guide, and U.S. Occupational Safety and Health Administration (OSHA) regulation, the 8 hour average respiratory exposure should not exceed 0.3 ppm. NIOSH recommends that the short term respiratory exposure to phosphine gas should not exceed 1 ppm. The Immediately Dangerous to Life or Health level is 50 ppm. Overexposure to phosphine gas causes nausea, vomiting, abdominal pain, diarrhea, thirst, chest tightness, dyspnea (breathing difficulty), muscle pain, chills, stupor or syncope, and pulmonary edema.[17][18] Phosphine has been reported to have the odor of decaying fish or garlic at concentrations below 0.3 ppm. The smell is normally restricted to laboratory areas or phosphine processing since the smell comes from the way the phosphine is extracted from the environment. However, it may occur elsewhere, such as in industrial waste landfills. Exposure to higher concentrations may cause olfactory fatigue.[19]
السمية
Deaths have resulted from accidental exposure to fumigation materials containing aluminum phosphide or phosphine.[20][21][22][23] It can be absorbed either by inhalation or transdermally.[20] As a respiratory poison, it affects the transport of oxygen or interferes with the utilization of oxygen by various cells in the body.[22] Exposure results in pulmonary edema (the lungs fill with fluid).[23] Phosphine gas is heavier than air so stays nearer the floor. [24]
Phosphine appears to be mainly a redox toxin, causing cell damage by inducing oxidative stress and mitochondrial dysfunction.[25] Resistance in insects is caused by a mutation in a mitochondrial metabolic gene.[15]
انظر أيضاً
- Diphosphane, H2PPH2, simplified to H4P2
- Diphosphines, R2PPR2, R2P(CH2)nPR2
- Diphosphene, HP=PH
- Diphosphenes, R-P=P-R'
- Phosphine oxide, R3P=O
- Phosphorane, PR5, R3P=CR2
- Phosphinite, P(OR)R2
- Phosphonite, P(OR)2R
- Phosphite, P(OR)3
- Phosphinate, R2P(RO)O
- Phosphonate, RP(RO)2O
- Phosphate, P(RO)3O
المراجع
- ^ Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (2017). Introduction to Organic Chemistry. New Delhi: Medtech (Scientific International, reprint of revised 4th edition, Macmillan, 1998). p. 828. ISBN 9789385998898.
- ^ Sennikov, P. G. (1994). "Weak H-Bonding by Second-Row (PH3, H2S) and Third-Row (AsH3, H2Se) Hydrides". Journal of Physical Chemistry. 98 (19): 4973–4981. doi:10.1021/j100070a006.
- ^ Toy, A. D. F. (1973). The Chemistry of Phosphorus. Oxford, UK: Pergamon Press.
- ^ Gokhale, S. D.; Jolly, W. L., "Phosphine", Inorganic Syntheses 1967, volume 9, pp. 56–58. doi:10.1002/9780470132401.ch17
- ^ Gassmann, G.; van Beusekom, J. E. E.; Glindemann, D. (1996). "Offshore atmospheric phosphine". Naturwissenschaften. 83 (3): 129–131. Bibcode:1996NW.....83..129G. doi:10.1007/BF01142178.
- ^ Roels, J.; Verstraete, W. (2001). "Biological formation of volatile phosphorus compounds, a review paper". Bioresource Technology. 79 (3): 243–250. doi:10.1016/S0960-8524(01)00032-3. PMID 11499578.
- ^ Kaplan, Sarah (July 11, 2016). "The first water clouds are found outside our solar system — around a failed star". The Washington Post. Retrieved September 14, 2020.
- ^ Chu, Jennifer (December 18, 2019). "A sign that aliens could stink". MIT News. Retrieved September 14, 2020.
- ^ Drake, Nadia (14 September 2020). "Possible sign of life on Venus stirs up heated debate". National Geographic. Retrieved 14 September 2020.
- ^ Greaves, J.S.; Richards, A.M.S.; Bains, W. (2020). "Phosphine gas in the cloud decks of Venus". Nature Astronomy. doi:10.1038/s41550-020-1174-4. Retrieved 14 September 2020.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ أ ب Stirone, Shannon; Chang, Kenneth; Overbye, Dennis (September 14, 2020). "Life on Venus? Astronomers See a Signal in Its Clouds". The New York Times. Retrieved September 14, 2020.
- ^ Burck, S.; Gudat, D.; Nieger, M.; Du Mont, W.-W. (2006). "P-Hydrogen-Substituted 1,3,2-Diazaphospholenes: Molecular Hydrides". Journal of the American Chemical Society. 128 (12): 3946–3955. doi:10.1021/ja057827j.
- ^ Trofimov, Boris A.; Arbuzova, Svetlana N.; Gusarova, Nina K. (1999). "Phosphine in the Synthesis of Organophosphorus Compounds". Russian Chemical Reviews. 68. doi:10.1070/RC1999v068n03ABEH000464.
- ^ Bettermann, G.; Krause, W.; Riess, G.; Hofmann, T. (2002). "Phosphorus Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_527.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ أ ب "A Core Metabolic Enzyme Mediates Resistance to Phosphine Gas". Science. 338: 807–810. 2012. Bibcode:2012Sci...338..807S. doi:10.1126/science.1224951. PMID 23139334.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "NIOSH Emergency Response Card". CDC. Retrieved 2010-04-06.
- ^ "NIOSH pocket guide". CDC. 2009-02-03. Retrieved 2010-04-06.
- ^ "WHO (Data Sheets on Pesticides-No. 46): Phosphine". Inchem.org. Archived from the original on 18 February 2010. Retrieved 2010-04-06.
- ^ "NIOSH Alert: Preventing Phosphine Poisoning and Explosions during Fumigation". CDC. 1995-07-10. Retrieved 2010-04-06.
- ^ أ ب "Two toddlers die after Jerusalem home sprayed for pests". Haaretz. 2014-01-22. Retrieved 2014-01-23.
- ^ rtve.es: "La familia de Alcalá de Guadaíra murió tras inhalar fosfina de unos tapones", 2014-02-03
- ^ أ ب "Deaths of Quebec women in Thailand may have been caused by pesticide". cbc.ca. 13 March 2014.
- ^ أ ب "4 children killed after pesticide released toxic gas underneath their home, police say". Washington Post. Retrieved 6 January 2017.
- ^ "Pesticide blamed in 8-month-old's death in Fort McMurray". CBC News. 2015-02-23. Retrieved 2015-02-23.
- ^ Nath, NS; Bhattacharya, I; Tuck, AG; Schlipalius, DI; Ebert, PR (2011). "Mechanisms of phosphine toxicity". Journal of toxicology. 2011: 494168. doi:10.1155/2011/494168. PMID 21776261.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
- E. Fluck, The chemistry of phosphine, Topics in Current Chemistry Vol. 35, 64 pp, 1973.
- WHO (World Health Organisation), Phosphine and selected metal phosphides, Environmental Health Criteria. Published under the joint sponsorship of UNEP, ILO and WHO, Geneva, Vol. 73, 100 pp, 1988.
وصلات خارجية
- CS1 errors: unsupported parameter
- CS1 errors: deprecated parameters
- CS1 maint: unflagged free DOI
- Articles with hatnote templates targeting a nonexistent page
- Missing redirects
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- مجموعات وظيفية
- فوسفينات
- غازات صناعية
- فوسفانات
- هيدريدات الفسفور
- مبخرات
- مركبات الفسفور(-3)
- Blood agents