مخيخ
| ||||||||||||||||||||
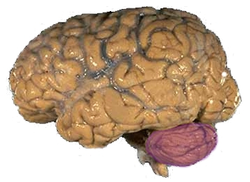
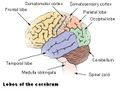
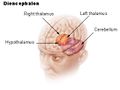
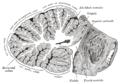
المخيخ Cerebellum هو القسم الكروي الأصغر الذي يقع أسفل نصفي الكرة المخيتين في الدماغ ، يهتم المخيخ بشكل أساسي بوظائف التوازن و تنظيم الوظائف الحركية. يقع أسفل النصفين الكرويين في الجهة الخلفية للمخ ويتركب من فصين أيمن وأيسر يصل بينهما فص ثالث.
cerebellum (Latin for "little brain") is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as or even larger.[1] In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses,[2][3] but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity.[4] Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.[4]
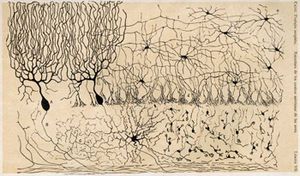
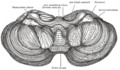
Anatomically, the human cerebellum has the appearance of a separate structure attached to the bottom of the brain, tucked underneath the cerebral hemispheres. Its cortical surface is covered with finely spaced parallel grooves, in striking contrast to the broad irregular convolutions of the cerebral cortex. These parallel grooves conceal the fact that the cerebellar cortex is actually a continuous thin layer of tissue tightly folded in the style of an accordion. Within this thin layer are several types of neurons with a highly regular arrangement, the most important being Purkinje cells and granule cells. This complex neural organization gives rise to a massive signal-processing capability, but almost all of the output from the cerebellar cortex passes through a set of small deep nuclei lying in the white matter interior of the cerebellum.[5]
In addition to its direct role in motor control, the cerebellum is necessary for several types of motor learning, most notably learning to adjust to changes in sensorimotor relationships. Several theoretical models have been developed to explain sensorimotor calibration in terms of synaptic plasticity within the cerebellum. These models derive from those formulated by David Marr and James Albus, based on the observation that each cerebellar Purkinje cell receives two dramatically different types of input: one comprises thousands of weak inputs from the parallel fibers of the granule cells; the other is an extremely strong input from a single climbing fiber.[6] The basic concept of the Marr–Albus theory is that the climbing fiber serves as a "teaching signal", which induces a long-lasting change in the strength of parallel fiber inputs. Observations of long-term depression in parallel fiber inputs have provided some support for theories of this type, but their validity remains controversial.[7]
البنية
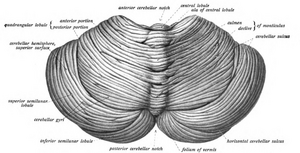
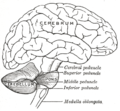
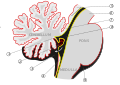
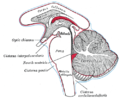
At the level of gross anatomy, the cerebellum consists of a tightly folded layer of cortex, with white matter underneath and a fluid-filled ventricle at the base. Four deep cerebellar nuclei are embedded in the white matter. Each part of the cortex consists of the same small set of neuronal elements, laid out in a highly stereotyped geometry. At an intermediate level, the cerebellum and its auxiliary structures can be separated into several hundred or thousand independently functioning modules called "microzones" or "microcompartments".
Gross anatomy
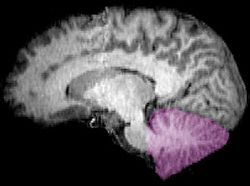
The cerebellum is located in the posterior cranial fossa. The fourth ventricle, pons and medulla are in front of the cerebellum.[8]
الأهمية الاكلينيكية
Damage to the cerebellum often causes motor-related symptoms, the details of which depend on the part of the cerebellum involved and how it is damaged. Damage to the flocculonodular lobe may show up as a loss of equilibrium and in particular an altered, irregular walking gait, with a wide stance caused by difficulty in balancing.[9] Damage to the lateral zone typically causes problems in skilled voluntary and planned movements which can cause errors in the force, direction, speed and amplitude of movements. Other manifestations include hypotonia (decreased muscle tone), dysarthria (problems with speech articulation), dysmetria (problems judging distances or ranges of movement), dysdiadochokinesia (inability to perform rapid alternating movements such as walking), impaired check reflex or rebound phenomenon, and intention tremor (involuntary movement caused by alternating contractions of opposing muscle groups).[10][11] Damage to the midline portion may disrupt whole-body movements, whereas damage localized more laterally is more likely to disrupt fine movements of the hands or limbs. Damage to the upper part of the cerebellum tends to cause gait impairments and other problems with leg coordination; damage to the lower part is more likely to cause uncoordinated or poorly aimed movements of the arms and hands, as well as difficulties in speed.[9] This complex of motor symptoms is called ataxia.
To identify cerebellar problems, neurological examination includes assessment of gait (a broad-based gait being indicative of ataxia), finger-pointing tests and assessment of posture.[4] If cerebellar dysfunction is indicated, a magnetic resonance imaging scan can be used to obtain a detailed picture of any structural alterations that may exist.[12]
The list of medical problems that can produce cerebellar damage is long, including stroke, hemorrhage, swelling of the brain (cerebral edema), tumors, alcoholism, physical trauma such as gunshot wounds or explosives, and chronic degenerative conditions such as olivopontocerebellar atrophy.[13][14] Some forms of migraine headache may also produce temporary dysfunction of the cerebellum, of variable severity.[15] Infection can result in cerebellar damage in such conditions as the prion diseases[16] and Miller Fisher syndrome, a variant of Guillain–Barré syndrome.
التقدم في العمر
يتغير المخيخ البشري مع التقدم في العمر. These changes may differ from those of other parts of the brain. The cerebellum is the youngest brain region (and body part) in centenarians according to an epigenetic biomarker of tissue age known as epigenetic clock: it is about 15 years younger than expected in a centenarian.[17] Further, gene expression patterns in the human cerebellum show less age-related alteration than that in the cerebral cortex.[18] Some studies have reported reductions in numbers of cells or volume of tissue, but the amount of data relating to this question is not very large.[19][20]
اضطرابات النمو والتنكس

Congenital malformation, hereditary disorders, and acquired conditions can affect cerebellar structure and, consequently, cerebellar function. Unless the causative condition is reversible, the only possible treatment is to help people live with their problems.[21] Visualization of the fetal cerebellum by ultrasound scan at 18 to 20 weeks of pregnancy can be used to screen for fetal neural tube defects with a sensitivity rate of up to 99%.[22]
In normal development, endogenous sonic hedgehog signaling stimulates rapid proliferation of cerebellar granule neuron progenitors (CGNPs) in the external granule layer (EGL). Cerebellar development occurs during late embryogenesis and the early postnatal period, with CGNP proliferation in the EGL peaking during early development (postnatal day 7 in the mouse).[23] As CGNPs terminally differentiate into cerebellar granule cells (also called cerebellar granule neurons, CGNs), they migrate to the internal granule layer (IGL), forming the mature cerebellum (by post-natal day 20 in the mouse).[23] Mutations that abnormally activate Sonic hedgehog signaling predispose to cancer of the cerebellum (medulloblastoma) in humans with Gorlin Syndrome and in genetically engineered mouse models.[24][25]
Congenital malformation or underdevelopment (hypoplasia) of the cerebellar vermis is a characteristic of both Dandy–Walker syndrome and Joubert syndrome.[26][27] In very rare cases, the entire cerebellum may be absent.[28] The inherited neurological disorders Machado–Joseph disease, ataxia telangiectasia, and Friedreich's ataxia cause progressive neurodegeneration linked to cerebellar loss.[13][21] Congenital brain malformations outside the cerebellum can, in turn, cause herniation of cerebellar tissue, as seen in some forms of Arnold–Chiari malformation.[29]
Other conditions that are closely linked to cerebellar degeneration include the idiopathic progressive neurological disorders multiple system atrophy and Ramsay Hunt syndrome type I,[30][31] and the autoimmune disorder paraneoplastic cerebellar degeneration, in which tumors elsewhere in the body elicit an autoimmune response that causes neuronal loss in the cerebellum.[32] Cerebellar atrophy can result from an acute deficiency of vitamin B1 (thiamine) as seen in beriberi and in Wernicke–Korsakoff syndrome,[33] or vitamin E deficiency.[21]
Cerebellar atrophy has been observed in many other neurological disorders including Huntington's disease, multiple sclerosis,[16] essential tremor, progressive myoclonus epilepsy, and Niemann–Pick disease. Cerebellar atrophy can also occur as a result of exposure to toxins including heavy metals or pharmaceutical or recreational drugs.[21]
الألم
There is a general consensus that the cerebellum is involved in pain processing.[34][35] The cerebellum receives pain input from both descending cortico-cerebellar pathways and ascending spino-cerebellar pathways, through the pontine nuclei and inferior olives. Some of this information is transferred to the motor system inducing a conscious motor avoidance of pain, graded according to pain intensity.
These direct pain inputs, as well as indirect inputs, are thought to induce long-term pain avoidance behavior that results in chronic posture changes and consequently, in functional and anatomical remodeling of vestibular and proprioceptive nuclei. As a result, chronic neuropathic pain can induce macroscopic anatomical remodeling of the hindbrain, including the cerebellum.[36] The magnitude of this remodeling and the induction of neuron progenitor markers suggest the contribution of adult neurogenesis to these changes.
صور اضافية
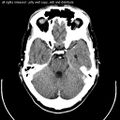
Computed tomography of head, with cerebellum visible at lower part
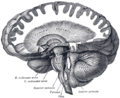
Dissection showing the course of the cerebrospinal fibers
المصادر
- ^ Hodos W (2009). "Evolution of Cerebellum". Encyclopedia of Neuroscience. Berlin, Heidelberg: Springer. pp. 1240–1243. doi:10.1007/978-3-540-29678-2_3124. ISBN 978-3-540-23735-8.
- ^ Wolf U, Rapoport MJ, Schweizer TA (2009). "Evaluating the affective component of the cerebellar cognitive affective syndrome". Journal of Neuropsychiatry and Clinical Neurosciences. 21 (3): 245–53. doi:10.1176/jnp.2009.21.3.245. PMID 19776302.
- ^ Schmahmann JD, Caplan D (February 2006). "Cognition, emotion and the cerebellum". Brain. 129 (Pt 2): 290–2. doi:10.1093/brain/awh729. PMID 16434422.
- ^ أ ب ت Fine EJ, Ionita CC, Lohr L (December 2002). "The history of the development of the cerebellar examination". Seminars in Neurology. 22 (4): 375–84. doi:10.1055/s-2002-36759. PMID 12539058.
- ^ Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, White LE (2011). Neuroscience (5th ed.). Sunderland, Mass.: Sinauer. pp. 417–423. ISBN 978-0-87893-695-3.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةAlbus - ^ Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, White LE (2007). Neuroscience (4th ed.). New York: W. H. Freeman. pp. 197–200. ISBN 978-0-87893-697-7.
- ^ Standring S, Borley NR, et al., eds. (2008). "Chapter 20". Gray's anatomy : the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. p. 297. ISBN 978-0-8089-2371-8.
- ^ أ ب Ghez C, Fahn S (1985). "The cerebellum". In Kandel ER, Schwartz JH (eds.). Principles of Neural Science, 2nd edition. New York: Elsevier. pp. 502–522.
- ^ Schmitz TJ (2007). "Examination of Coordination". In O'Sullivan SB, Schmitz TJ (eds.). Physical Rehabilitation. Philadelphia: F. A. Davis. pp. 193–225. ISBN 9780803612471.
- ^ Mariën P, Manto M (2016). The linguistic cerebellum. London, UK: Academic Press. pp. 337–351. ISBN 978-0-12-801608-4.
- ^ Gilman S (March 1998). "Imaging the brain. Second of two parts". New England Journal of Medicine. 338 (13): 889–96. doi:10.1056/NEJM199803263381307. PMID 9516225.
- ^ أ ب "NINDS Ataxias and Cerebellar or Spinocerebellar Degeneration Information Page". National Institutes of Health. 16 April 2014. Archived from the original on 9 February 2015. Retrieved 2 February 2015.
- ^ Yuhas D (January 15, 2016). "Veterans of Iraq, Afghanistan Show Brain Changes Related to Explosion Exposure". Scientific American. Archived from the original on January 20, 2016. Retrieved January 21, 2016.
- ^ Vincent M, Hadjikhani N (June 2007). "The cerebellum and migraine". Headache. 47 (6): 820–33. doi:10.1111/j.1526-4610.2006.00715.x. PMC 3761082. PMID 17578530.
- ^ أ ب "NINDS Cerebellar Degeneration Information Page". National Institutes of Health. 28 February 2014. Archived from the original on 18 February 2015. Retrieved 2 February 2015.
- ^ Horvath S, Mah V, Lu AT, Woo JS, Choi OW, Jasinska AJ, Riancho JA, Tung S, Coles NS, Braun J, Vinters HV, Coles LS (May 2015). "The cerebellum ages slowly according to the epigenetic clock". Aging. 7 (5): 294–306. doi:10.18632/aging.100742. PMC 4468311. PMID 26000617.
- ^ Fraser HB, Khaitovich P, Plotkin JB, Pääbo S, Eisen MB (September 2005). "Aging and gene expression in the primate brain". PLOS Biology. 3 (9): e274. doi:10.1371/journal.pbio.0030274. PMC 1181540. PMID 16048372.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Andersen BB, Gundersen HJ, Pakkenberg B (November 2003). "Aging of the human cerebellum: a stereological study". Journal of Comparative Neurology. 466 (3): 356–65. doi:10.1002/cne.10884. PMID 14556293. S2CID 7091227.
- ^ Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD (2001). "Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults" (PDF). American Journal of Neuroradiology. 22 (6): 1161–7. PMC 7974784. PMID 11415913. Archived (PDF) from the original on 2008-12-17.
- ^ أ ب ت ث Albert RK, Porter RS, eds. (2006). The Merck Manual of Diagnosis and Therapy (18th ed.). Whitehouse Station, New Jersey: Merck Research Libraries. pp. 1886–1887.
- ^ Campbell J, Gilbert WM, Nicolaides KH, Campbell S (August 1987). "Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population". Obstetrics and Gynecology. 70 (2): 247–50. PMID 3299184.
- ^ أ ب Hatten ME, Heintz N (1995). "Mechanisms of neural patterning and specification in the developing cerebellum". Annual Review of Neuroscience. 18: 385–408. doi:10.1146/annurev.ne.18.030195.002125. PMID 7605067.
- ^ Polkinghorn WR, Tarbell NJ (May 2007). "Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification". Nature Clinical Practice. Oncology. 4 (5): 295–304. doi:10.1038/ncponc0794. PMID 17464337. S2CID 24461280.
- ^ Roussel MF, Hatten ME (2011). Cerebellum development and medulloblastoma. Vol. 94. pp. 235–82. doi:10.1016/B978-0-12-380916-2.00008-5. ISBN 9780123809162. PMC 3213765. PMID 21295689.
{{cite book}}:|journal=ignored (help) - ^ "NINDS Joubert Syndrome Information Page". National Institutes of Health. 23 December 2013. Archived from the original on 4 January 2015. Retrieved 9 January 2015.
- ^ "NINDS Dandy-Walker Information Page". National Institutes of Health. 14 February 2014. Archived from the original on 4 January 2015. Retrieved 9 January 2015.
- ^ "NINDS Cerebellar Hypoplasia Information Page". National Institutes of Health. 29 September 2011. Archived from the original on 4 January 2015. Retrieved 9 January 2015.
- ^ "Chiari Malformation Fact Sheet". National Institutes of Health. 10 December 2014. Archived from the original on 27 October 2011. Retrieved 9 January 2015.
- ^ "NINDS Dyssynergia Cerebellaris Myoclonica Information Page". National Institutes of Health. 14 February 2011. Archived from the original on 16 February 2015. Retrieved 1 February 2015.
- ^ "NINDS Olivopontocerebellar Atrophy Information Page". National Institutes of Health. 16 April 2014. Archived from the original on 27 January 2012. Retrieved 9 January 2015.
- ^ "NINDS Paraneoplastic Syndromes Information Page". National Institutes of Health. 12 March 2009. Archived from the original on 4 January 2015. Retrieved 9 January 2015.
- ^ "NINDS Wernicke-Korsakoff Syndrome Information Page". National Institutes of Health. 14 February 2007. Archived from the original on 4 January 2015. Retrieved 9 January 2015.
- ^ Moulton EA, Schmahmann JD, Becerra L, Borsook D (October 2010). "The cerebellum and pain: passive integrator or active participator?". Brain Research Reviews. 65 (1): 14–27. doi:10.1016/j.brainresrev.2010.05.005. PMC 2943015. PMID 20553761.
- ^ Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA (April 2015). "Consensus paper: the role of the cerebellum in perceptual processes". Cerebellum. 14 (2): 197–220. doi:10.1007/s12311-014-0627-7. PMC 4346664. PMID 25479821.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةReferenceA
للاستزادة
- Ito M. Cerebellum and Neural Control. New York: Raven Press; 1984. ISBN 0-89004-106-7
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed. McGraw-Hill, New York (2000). ISBN 0-8385-7701-6
- Llinás, R, Sotelo C. The Cerebellum Revisited. New York: Springer; 1992. ISBN 0-387-97693-0
- Parent A, Carpenter MB. Carpenter's Human Neuroanatomy. 9th ed. Philadelphia: Williams and Wilkins; 1995. ISBN 0-683-06752-4
وصلات خارجية
- A worldwide list of laboratories that do research on the cerebellum at the Internet Archive
- Cerebellum and multiple sclerosis at mult-sclerosis.org
- Basal ganglia and cerebellum at Washington University in St. Louis
- Cerebellum - Cell Centered Database
- "The Treasure at the Bottom of the Brain” at newhorizons.org
- Cerebellum images at About.com
- قالب:BrainMaps
- Histological section of primate cerebellum at University of Birmingham
- Cerebellum and Motor Control, Neurophysiology Lecture Site, Ronald E. Young, Ph.D., University of West Indies, Mona.
- Comparative Neuroscience at Wikiversity
- NIF Search - Cerebellum via the Neuroscience Information Framework